Parthenocarpy
- 1. Parthenocarpy Mandeep Kaur Ph.D. Fruit Science
- 2. Parthenocarpy Parthenocarpy (literally meaning a virgin fruit) is the natural or artificially induced production of fruit without fertilization of ovules. The fruit is therefore seedless (Noll, 1902). Parthenogenesis refers to production of egg cells without fertilization. E.g. Mangosteen.
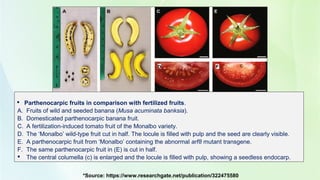
- 3. Parthenocarpic fruits in comparison with fertilized fruits. A. Fruits of wild and seeded banana (Musa acuminata banksia). B. Domesticated parthenocarpic banana fruit. C. A fertilization-induced tomato fruit of the Monalbo variety. D. The ‘Monalbo’ wild-type fruit cut in half. The locule is filled with pulp and the seed are clearly visible. E. A parthenocarpic fruit from ‘Monalbo’ containing the abnormal arf8 mutant transgene. F. The same parthenocarpic fruit in (E) is cut in half. The central columella (c) is enlarged and the locule is filled with pulp, showing a seedless endocarp. *Source: https://www.researchgate.net/publication/322475580
- 4. Examples of seeded and seedless fruits in parthenocarpic species A.Annona squamosa B.Actinidia arguta cv. Issai C.Cucumis sativus D.Elaeis oleifera (African oil palm) E.Citrus clementine Source: https://www.frontiersin.org/articles/10.3389/fpls.2018.01997/full A B C D E
- 5. Importance of parthenocarpy Seedlessness is a desirable trait in edible fruits with hard seeds (pineapple, banana, orange, grapefruit). Also desirable in fruit crops that may be difficult to pollinize or fertilize. In dioecious species (e.g. Persimmon), pathenocarpy increases fruit production as staminate trees do not need to be planted to provide pollen. Increases shelf life due to reduced ethylene generated by seeds. Improves processing quality & ultimately increases profitability. Undesirable effects of parthenocarpy Parthenocarpy is undesirable in nuts (e.g. Pistachio, walnut, almond & pecan). It occasionally occurs as a mutation in nature & is considered as a defect because the plant can no longer sexually reproduce.
- 6. Types of Parthenocarpy Natural or Genetic Artificial or Induced *Source: Gorguet et al., 2005
- 7. Triploid banana The triploid banana fruit is vegetatively parthenocarpic. The graphs of growth in volume of seeded banana fruits are sigmoid in shape. Those of parthenocarpic fruits are variable but are not sigmoid and the shapes are related to specific origins. Growth rates are related to certain ovule behaviours, to seed content of the fruit, and to ploidy. NAA induces parthenocarpy in seeded bananas and stimulates it in weakly parthenocarpic types. By contrast, coumarin, a hormone inhibitor, inhibits it in strongly parthenocarpic forms.
- 8. Pollinated & Parthenocarpic Fig B C *Source https://www.frontiersin.org/articles/10.3389/fpls.2016.01696/full
- 9. Stenospermocarpic Grapes Normal pollination and fertlization occurs, but the embryos abort when they are young. Often, remnants of seeds can be seen in the fruits. Characteristic feature of the group of sultana - type raisin cultivars, for example Kishmish rozovyi (Pink Sultana), Black Kishmish, Black Monukka and Thompson Seedless grapes.
- 10. Origin of parthenocarpy Due to absence of pollination and fertilization Due to male sterility Degradation of pollen mother cells Degradation of fertilized ovules Due to self-incompatibility e.g. Citrus Due to embryo abortion e.g. Grape Due to chromosomal irregularities during meiosis leading to triploidy e.g. Tahiti lime, Oroblanco and Melogold.
- 11. Physiological basis of parthenocarpy To obtain parthenocarpic fruits is a physiological challenge. Fruit development comprises early development & maturation. Early fruit development in most of the plants involves three phases: (i). fruit setting (ii). Cell division (iii). Cell expansion. The ovary takes decision to either abort or to go further in fruit development during first phase. During cell division phase of growth of fruit takes place, however, the increase in fruit size is low because the dividing cells are small and tightly compressed & the number of cells decides final fruit size. The third & last phase begins after cell division stops, the fruit grows by the increase in cell volume, until it reaches its final size. Cell expansion commonly increases fruit size by 100 folds, & this makes the greatest contribution to the final fruit size.
- 13. Working hypothesis of parthenocarpic fruit set Auxin induces parthenocarpy The ovaries of parthenocarpic fruits have higher content of auxin than corresponding non - parthenocarpic cultivars.
- 14. Stenospermocarpy Floral development Defect in embryo or endosperm development Pollination/ fertilization Fruit development without seed Parthenocarpy Floral development Post-pollination floral development Ovule development arrest Fruit development without seed Apomixis Floral development Post-pollination floral development Ovule development arrest Autonomous fruit & seed development Floral development Senescence Embryo, endosperm & integument growth Pollination/ fertilization Fruit & seed development Fertilization Comparison between Fertilization, Parthenocarpy, Stenospermocary & Apomixis
- 15. Causes & induction of parthenocarpy in fruit crops Fruit crops Causes Guava e.g. Allahabad Seedless • Due to triploidy • Failure of fertilization of well differentiated ovules • Degeneration of fertilized ovules Grape • Due to embryo abortion (stenospermocarpy) Citrus • Due to self-incompatibility (mandarins) • Cytoplasmic male sterility (Satsuma Mandarin) • Chromosomal irregularities (Tahiti lime, Oroblanco, Melogold) Apple • Lack of pollination Banana • Due to triploidy
- 16. Papaya Rodriquez – Pastor et al. (1990) have observed parthenocarpy at rate of 35% in Sunrise and 5 % in 298 F5. Parthenocarpic fruits are of adequate quality. Litchi ‘Chicken- tongue’ seeds to the shriveled seeds with rudimentary embroys. The percentage of fruits having chicken - tongue seeds differ with the cultivars, 8.4% in Shahi to over 90% in Bedana. Banana Seed sterility is due to cytogenetic factors and triploidy. Parthenocarpy is due to several (at least three) complementary dominant genes (P1, P2 and P3) which are present in wild forms of Musa acuminata (Simmonds, 1976). Some modifier genes are also responsible for parthenocarpy.
- 17. Citrus Low pollen viability and a high degree of ovule sterility. In ‘Tahiti Lime’ and ‘Oroblanco’ : chromosomal irregularities during meiosis (Triploidy). ‘Wilking’ Mandarin : pollen, as well as ovule, abortion and subsequent parthenocarpy. Apple CPPU, GA₃ and GA7 had a positive synergistic effect on parthenocarpic fruit production in apple cultivars Golden Delicious and Jonagold. Pear GA3 and GA 4+7 induce parthenocarpy when applied before full bloom. In cv. Rocha, only a few pollen tubes reached the ovules 8 days after self pollination. The slow rate of pollen tube growth in self-pollination of Rocha pear may prevent fertilisation by loss of viability of embryo sacs (Medeira and Maia 2008).
- 18. Changes in physico-chemical characteristics in the fruits due to induction of parthenocarpy Size: In mango seedless fruits had smaller size than seeded fruits but had good quality and matured earlier than seeded fruits. In grapes also the size of seedless berries was smaller than the seeded ones of the same variety. Form: Parthenocarpic fruits (induced by GA) in apple were usually more elongated than the normal seeded fruits. Composition and quality: In most grape varieties, seedless fruits are sweeter than the seeded fruits of the same variety. Maturity: Seedless fruits ripen later than seeded fruits which may be attributed to ethylene produced by seeds.
- 19. Factors affecting/ influencing parthenocarpy Environmental Factors (High or low temperature). Chromosomal aberration/ genetic disorder. Growth regulators. Self- Incompatibility. Genetic factors like gene controlling meiosis.
- 20. Biotechnological approaches to induce parthenocarpy Due to induction of male sterility. Seed coat destruction by suicide gene expression. Induction of female sterility- destruction of ovules or stigma by suicide genes. Increased expression or sensitivity to GA and auxins in ovary.
- 21. Role of growth regulators in parthenocarpy Auxin, gibberellins and cytokinins or mixtures of these are effective in inducing fruit in several crop species. Auxin and gibberellin may act in parallel or in a sequential way on fruit set. Auxin Natural and artificial auxins supplied exogenously to unpollinated flowers induce fruit growth in tomato and in other horticultural plants. It can replace the signals provided by pollination and fertilization. Gibberellins Active gibberellins (GA₁ or GA₃) are able to induce fruit set parthenocarpically (Pandolfini, 2009). Increased levels of gibberellin in pollinated ovary & an increased expression of GA biosynthetic genes (Serrani et al., 2009).
- 22. Ethylene In unpollinated tomato ovaries ethylene biosynthesis and signalling genes are highly expressed and it is known that in many plant species pollination itself triggers a transient increase in ethylene production in pistils. Plays a role in coordinating ovary growth and flower senescence events that are associated to fruit set. Brassinosteroids (BRs) Interact with auxins synergistically. Parthenocarpic growth in cucumber: active cell division. Application of an inihibitor of BRs biosynthesis blocked fruit growth in a parthenocarpic cultivar.
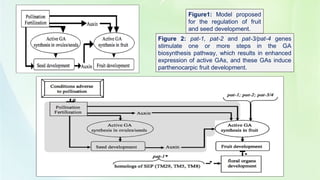
- 23. Figure1: Model proposed for the regulation of fruit and seed development. Figure 2: pat-1, pat-2 and pat-3/pat-4 genes stimulate one or more steps in the GA biosynthesis pathway, which results in enhanced expression of active GAs, and these GAs induce parthenocarpic fruit development.
- 24. Target Cause/treatment Underlying pathway Species Source Auxin (NAA) Exogenous application Auxin Strawberry (Fragaria ananassa and F. vesca) Nitsch (1950); Kang et al. (2013) YUCCA Overexpression Auxin Eriobotrya japonica Mesejo et al. (2010) ARF-7/8 Underexpression Auxin A. thaliana; S. lycopersicum Goetz et al. (2007); de Jong et al. (2009) Gibberellic acid Exogenous application GA M. domestica; F. vesa Davison (1960); Prosser and Jackson (1959); Kang et al. (2013) GA20OX Overexpression GA A. thaliana; S. lycopersicum García-Hurtado et al. (2012) DELLA RNAi; loss-of-function mutations GA A. thaliana; S. lycopersicum Martí et al. (2007); Fuentes et al. (2012) Genes and phytohormones involved in parthenocarpic fruit development
- 25. Genes and phytohormones involved in parthenocarpic fruit development Target Cause/treatment Underlying pathway Species Source Cytokinin Exogenous application Cytokinin A. deliciosa Hayata and Niimi (1995); Lewis et al. (1996) MET1 RNAi DNA methylation A. thaliana FitzGerald et al. (2008); Schmidt et al. (2013) PISTILLAT A Gain- and loss-of-function mutations MADS-box Malus domesticus; Vitis vinifera Yao et al. (2001); Fernandez et al. (2013) DEFICIENS Loss-of-function mutation MADS-box Elaeis guineensis Ong-Abdullah et al. (2015) SEP1/TM29 Antisense or co- suppression MADS-box S. lycopersicum Ampomah-Dwamena et al. (2002)
- 26. Pollen effects on fruit set, seed weight, & shriveling of ‘73-S-20’ litchi- with special reference to artificial induction of parthenocarpy (Chu et al., 2015) Various types of seeded fruit in ‘73-S-20’ litchi: (A). Three types of seeded fruits simultaneously hang within a cluster. Normal-seeded (a) developed by double fertization Shriveled seed (b) developed by stenospermocarpy Small seedless fruit (c) developed by partheoncarpy in the same cluster. (B). Many small parthenocarpic fruit hang in a cluster accompanied by normal-sized fruits. aa bb cc Source: https://journals.ashs.org/hortsci/view/journals/hortsci/50/3/article-p369.xml
- 27. Pollen source Fruit set (%) Fruit weight (g) Aril weight (g) Seed weight (g) 73-S-20 (Self pollinated) 10.3 15.9 11.8 1.1 Haak Yip (Cross pollinated) 15.8 17.4 12.3 1.9 Non-pollinated 21.3 4.2 2.9 0.0 Three types of fruits in ‘73-S-20’ litchi : (A)Normal fruits with normal seeds. (B)Normal fruits with shriveled seed. (C)Parthenocarpic fruits with no seeds.
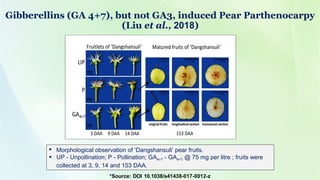
- 28. Gibberellins (GA 4+7), but not GA3, induced Pear Parthenocarpy (Liu et al., 2018) Morphological observation of ‘Dangshansuli’ pear fruits. UP - Unpollination; P - Pollination; GA4+7 - GA4+7 @ 75 mg per litre ; fruits were collected at 3, 9, 14 and 153 DAA.
- 29. The mechanistic model of fruit set. Gene names in red font represent related genes were upregulated. Instead, those in green front represent related genes were downregulated. *Source: DOI 10.1038/s41438-017-0012-z
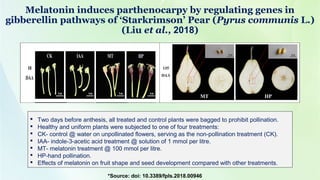
- 30. Melatonin induces parthenocarpy by regulating genes in gibberellin pathways of ‘Starkrimson’ Pear (Pyrus communis L.) (Liu et al., 2018) Two days before anthesis, all treated and control plants were bagged to prohibit pollination. Healthy and uniform plants were subjected to one of four treatments: CK- control @ water on unpollinated flowers, serving as the non-pollination treatment (CK). IAA- indole-3-acetic acid treatment @ solution of 1 mmol per litre. MT- melatonin treatment @ 100 mmol per litre. HP-hand pollination. Effects of melatonin on fruit shape and seed development compared with other treatments. *Source: doi: 10.3389/fpls.2018.00946
- 31. Model depicting the mechanism of gibberellins (GAs) in melatonin-mediated fruit set in ‘Starkrimson’ pear. Exogenous melatonin is involved in plant hormone metabolism, and melatonin regulates the process of parthenocarpy through the GA-synthesis pathway. GA20ox and GA2ox genes are mainly regulated to increase GA content, which promotes fruit set, and melatonin triggers cell division and expansion of the mesocarp to form fruit through induced GA biosynthesis. Conti…
- 32. Induction of parthenocarpy by bordeaux mixture and GA and its use for cultivation in Japanese Pear ‘Kosui’ (Hayashida et al., 2016) Copper (Cu2+ ) and ferrous (Fe2+ ) ions. Parthenocarpic fruits formed, because : Self-pollen tube growth was not promoted by Cu 2+ and Fe2+.. Almost no perfect seeds were observed at harvest. Cu2+ and Fe2+ acted as strong inhibitors of pollen tube growth in vitro. The growth of Bordeaux mixture-induced fruit was improved by gibberellin (GA) paste or GA paste mixed with N-(N-(2-chloro-4-pyridy1)-N'-phenylurea (CPPU) treatment of the fruit stalk; the treated fruit was about 100 g heavier than the untreated fruit.
- 33. Effects of exogenous application of GA4+7 and N-(2-chloro-4-pyridyl)-N′- phenylurea on induced parthenocarpy and fruit quality in Pyrus pyrifolia ‘Cuiguan’ (Niu et al., 2014) Morphological changes in fruit of ‘Cuiguan’ pear as affected by GA 4+7 and/or CPPU treatments and hand pollination and transverse sections of ripe fruit induced by hand pollination and 500 mg per litre GA 4+7. P - Hand pollination. C - CPPU @ 30 mg per litre.₃₀ G ₃₀₀C - GA₃₀ 4+7 @ 300 mg per litre & CPPU @ 30 mg per litre. G - GA₅₀₀ 4+7 @ 500 mg per litre. *Source: https://www.researchgate.net/publication/279220212
- 34. Future thrusts High level and stable parthenocarpy Combining several parthenocarpy genes Developing parthenocarpy in high value crops Combining parthenocarpy with male sterility