periodic_table.ppt
- 1. Periodic Table of Elements
- 4. Elements Science has come along way since Aristotle’s theory of Air, Water, Fire, and Earth. Scientists have identified 90 naturally occurring elements, and created about 28 others.
- 5. Elements The elements, alone or in combinations, make up our bodies, our world, our sun, and in fact, the entire universe.
- 6. The most abundant element in the earth’s crust is oxygen.
- 7. Periodic Table The periodic table organizes the elements in a particular way. A great deal of information about an element can be gathered from its position in the period table. For example, you can predict with reasonably good accuracy the physical and chemical properties of the element. You can also predict what other elements a particular element will react with chemically. Understanding the organization and plan of the periodic table will help you obtain basic information about each of the 118 known elements.
- 8. Key to the Periodic Table Elements are organized on the table according to their atomic number, usually found near the top of the square. The atomic number refers to how many protons an atom of that element has. For instance, hydrogen has 1 proton, so it’s atomic number is 1. The atomic number is unique to that element. No two elements have the same atomic number.
- 9. What’s in a square? Different periodic tables can include various bits of information, but usually: atomic number symbol atomic mass number of valence electrons state of matter at room temperature.
- 10. Atomic Number This refers to how many protons an atom of that element has. No two elements, have the same number of protons. Bohr Model of Hydrogen Atom Wave Model
- 11. Atomic Mass Atomic Mass refers to the “weight” of the atom. It is derived at by adding the number of protons with the number of neutrons. H This is a helium atom. Its atomic mass is 4 (protons plus neutrons). What is its atomic number?
- 12. View CD-ROM Atoms and Elements
- 13. Atomic Mass and Isotopes While most atoms have the same number of protons and neutrons, some don’t. Some atoms have more or less neutrons than protons. These are called isotopes. An atomic mass number with a decimal is the total of the number of protons plus the average number of neutrons.
- 14. Atomic Mass Unit (AMU) The unit of measurement for an atom is an AMU. It stands for atomic mass unit. One AMU is equal to the mass of one proton.
- 15. Atomic Mass Unit (AMU) There are 6 X 1023 or 600,000,000,000,000, 000,000,000 amus in one gram. (Remember that electrons are 2000 times smaller than one amu).
- 16. Symbols All elements have their own unique symbol. It can consist of a single capital letter, or a capital letter and one or two lower case letters. C Carbon Cu Copper
- 18. Valence Electrons The number of valence electrons an atom has may also appear in a square. Valence electrons are the electrons in the outer energy level of an atom. These are the electrons that are transferred or shared when atoms bond together.
- 20. Properties of Metals Metals are good conductors of heat and electricity. Metals are shiny. Metals are ductile (can be stretched into thin wires). Metals are malleable (can be pounded into thin sheets). A chemical property of metal is its reaction with water which results in corrosion.
- 21. Properties of Non-Metals Non-metals are poor conductors of heat and electricity. Non-metals are not ductile or malleable. Solid non-metals are brittle and break easily. They are dull. Many non-metals are gases. Sulfur
- 22. Properties of Metalloids Metalloids (metal-like) have properties of both metals and non-metals. They are solids that can be shiny or dull. They conduct heat and electricity better than non- metals but not as well as metals. They are ductile and malleable. Silicon
- 25. Families Periods Columns of elements are called groups or families. Elements in each family have similar but not identical properties. For example, lithium (Li), sodium (Na), potassium (K), and other members of family IA are all soft, white, shiny metals. All elements in a family have the same number of valence electrons. Each horizontal row of elements is called a period. The elements in a period are not alike in properties. In fact, the properties change greatly across even given row. The first element in a period is always an extremely active solid. The last element in a period, is always an inactive gas.
- 38. Hydrogen The hydrogen square sits atop Family AI, but it is not a member of that family. Hydrogen is in a class of its own. It’s a gas at room temperature. It has one proton and one electron in its one and only energy level. Hydrogen only needs 2 electrons to fill up its valence shell.
- 39. Alkali Metals The alkali family is found in the first column of the periodic table. Atoms of the alkali metals have a single electron in their outermost level, in other words, 1 valence electron. They are shiny, have the consistency of clay, and are easily cut with a knife.
- 40. Alkali Metals They are the most reactive metals. They react violently with water. Alkali metals are never found as free elements in nature. They are always bonded with another element.
- 41. What does it mean to be reactive? We will be describing elements according to their reactivity. Elements that are reactive bond easily with other elements to make compounds. Some elements are only found in nature bonded with other elements. What makes an element reactive? An incomplete valence electron level. All atoms (except hydrogen) want to have 8 electrons in their very outermost energy level (This is called the rule of octet.) Atoms bond until this level is complete. Atoms with few valence electrons lose them during bonding. Atoms with 6, 7, or 8 valence electrons gain electrons during bonding.
- 42. 5
- 45. Alkaline Earth Metals They are never found uncombined in nature. They have two valence electrons. Alkaline earth metals include magnesium and calcium, among others.
- 46. Transition Metals Transition Elements include those elements in the B families. These are the metals you are probably most familiar: copper, tin, zinc, iron, nickel, gold, and silver. They are good conductors of heat and electricity.
- 47. Transition Metals The compounds of transition metals are usually brightly colored and are often used to color paints. Transition elements have 1 or 2 valence electrons, which they lose when they form bonds with other atoms. Some transition elements can lose electrons in their next-to-outermost level.
- 48. Transition Elements Transition elements have properties similar to one another and to other metals, but their properties do not fit in with those of any other family. Many transition metals combine chemically with oxygen to form compounds called oxides.
- 49. Boron Family The Boron Family is named after the first element in the family. Atoms in this family have 3 valence electrons. This family includes a metalloid (boron), and the rest are metals. This family includes the most abundant metal in the earth’s crust (aluminum).
- 50. Carbon Family Atoms of this family have 4 valence electrons. This family includes a non-metal (carbon), metalloids, and metals. The element carbon is called the “basis of life.” There is an entire branch of chemistry devoted to carbon compounds called organic chemistry.
- 51. Nitrogen Family The nitrogen family is named after the element that makes up 78% of our atmosphere. This family includes non- metals, metalloids, and metals. Atoms in the nitrogen family have 5 valence electrons. They tend to share electrons when they bond. Other elements in this family are phosphorus, arsenic, antimony, and bismuth.
- 52. Oxygen Family Atoms of this family have 6 valence electrons. Most elements in this family share electrons when forming compounds. Oxygen is the most abundant element in the earth’s crust. It is extremely active and combines with almost all elements.
- 53. Halogen Family The elements in this family are fluorine, chlorine, bromine, iodine, and astatine. Halogens have 7 valence electrons, which explains why they are the most active non- metals. They are never found free in nature. Halogen atoms only need to gain 1 electron to fill their outermost energy level. They react with alkali metals to form salts.
- 54. Noble Gases Noble Gases are colorless gases that are extremely un- reactive. One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. The family of noble gases includes helium, neon, argon, krypton, xenon, and radon. All the noble gases are found in small amounts in the earth's atmosphere.
- 55. Rare Earth Elements The thirty rare earth elements are composed of the lanthanide and actinide series. One element of the lanthanide series and most of the elements in the actinide series are called trans-uranium, which means synthetic or man-made.
- 56. Mendeleev In 1869, Dmitri Ivanovitch Mendeléev created the first accepted version of the periodic table. He grouped elements according to their atomic mass, and as he did, he found that the families had similar chemical properties. Blank spaces were left open to add the new elements he predicted would occur.
- 57. Matter All matter is composed of atoms and groups of atoms bonded together, called molecules. Substances that are made from one type of atom only are called pure substances. Substances that are made from more than one type of atom bonded together are called compounds. Compounds that are combined physically, but not chemically, are called mixtures.
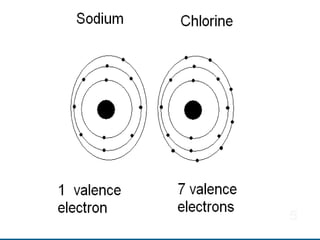
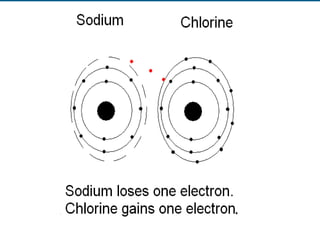
- 58. Elements, Compounds, Mixtures Sodium is an element. Chlorine is an element. When sodium and chlorine bond they make the compound sodium chloride, commonly known as table salt. Compounds have different properties than the elements that make them up. Table salt has different properties than sodium, an explosive metal, and chlorine, a poisonous gas.
- 59. Elements, Compounds, Mixtures Hydrogen is an element. Oxygen is an element. When hydrogen and oxygen bond they make the compound water. When salt and water are combined, a mixture is created. Compounds in mixtures retain their individual properties. The ocean is a mixture.
- 60. Elements, compounds, and mixtures Mixtures can be separated by physical means. Compounds can only be separated by chemical means. Elements are pure substances. When the subatomic particles of an element are separated from its atom, it no longer retains the properties of that element.