Perivascular Epithelioid Cell Tumors ( PEComa) of GIT
- 1. PERIVASCULAR EPITHELIOID CELL TUMORS (PECOMA) OF GIT Dr. Reenaz Shaik MD Pathology
- 2. CONTENTS Introduction History PEComa family Epidemiology Etiology Clinical features Macroscopic findings Microscopic findings and Classification Electron microscopic findings FISH, PCR, DNA sequencing, Genetic findings Differential Diagnosis Management and follow up
- 3. INTRODUCTION Perivascular epithelioid cell tumors (PEComas) are rare mesenchymal neoplasm with distinctive morphology and immunohistochemical characteristics. They most often arise in the retro peritoneum, visceral organs and abdomino-pelvic sites. These tumors usually show reactivity for melanocytic and smooth muscle markers HMB45, Melan-A, MiTF, smooth muscle actin (SMA) and calponin.
- 4. HISTORY In 1992, Bonetti et al., firstly created the term of perivascular epithelioid cell (PEC) to portray some epithelioid type cells with a perivascular distribution and immunoreactive for melanocyte markers. In 2002, the World Health Organization took the definition of “mesenchymal tumors composed of histologically and immunohistochemically distinctive perivascular epithelioid cells” as perivascular epithelioid cell tumors (PEComas).
- 5. PECOMA FAMILY Includes 1. Angiomyolipoma (AML) 2. Clear cell “sugar” tumor of the lung (CCST) 3. Lymphangioleiomyomatosis (LAM) 4. Clear cell myomelanocytic tumor of the falciform ligament/ligamentum teres (CCMMT) 5. Unusual clear cell tumors in other locations (uterus, colon, and soft tissues) (NOS)
- 6. Gastrointestinal tract (GI) is one of the most common anatomic sites of origin and counts for 20% to 25% of all reported cases of perivascular epithelioid cell tumors not otherwise specified (PEComas-NOS). PEComas have been reported in various anatomic sites with a marked female predominance.
- 7. EPIDEMIOLOGY Rare tumor. There are about 50 cases of GI PEComas- NOS according to the literatures so far. GI is the second most frequent site of PEComas, only behind gynecological tract. The age of patients who were diagnosed with GI PEComas-NOS ranging from 5.5 to 97 years with an average age of 38.9. The most frequently involved location is colon. Strong association with Tuberous sclerosis is seen.
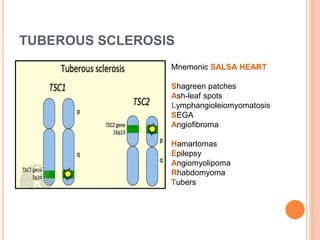
- 8. PECOMA AND TUBEROUS SCLEROSIS PEComas are related to the tuberous sclerosis complex (TSC) or to the genetic alterations of TSC. TS is an autosomal dominant genetic disease due to losses of TSC1 (9q34) or TSC2 (16p13.3) genes Characterized by: - Mental retardation - Seizures - Cellular proliferations (AMLs, subependymal giant cell tumours, cutaneous angiofibroma's, cardiac rhabdomyomas, micronodular hyperplasia, lymphangioleiomyomatosis, pulmonary multifocal micronodular hyperplasia)
- 9. TUBEROUS SCLEROSIS Mnemonic SALSA HEART Shagreen patches Ash-leaf spots Lymphangioleiomyomatosis SEGA Angiofibroma Hamartomas Epilepsy Angiomyolipoma Rhabdomyoma Tubers
- 10. ETIOLOGY The exact cause of GI PEComas-NOS is not clear. Theories: 1. Originate from PEC but the natural histological counterpart is out of clarity. 2. From neural crest for their expressing many melanocytic and muscular markers and as they are ubiquitous. 3. From smooth muscle with possible molecular alteration that brings to expression of melanogenesis and melanocytic markers. 4. From telocyte as markers described in telocytes (CD34, S-100, smooth muscle actin, and vascular endothelial growth factor) are positive.
- 11. CLINICAL FEATURES GI PEComa-NOS presents with non-specific clinical signs. With symptoms relying on the different organ involved, the size of neoplasm and tumor volume, principal clinical presentations. Abdominal pain, melaena, rectal bleeding, obstruction, weight loss, anemia, and some even asymptomatic. Abdominal pain is most frequent (35%) that may result from oppression, impaction, and hemorrhage.
- 12. LOCATISATION PEComas of the digestive tract frequently arise from colon, liver, small intestine, pancreas. Also seen in mesentery and peritoneum Very rare in stomach Angiomyolipoma is a subtype that contains adipose tissue and tortuous and thick walled vessels almost exclusively is present in liver, kidney and pancreas.
- 13. ENDOSCOPY Endoscopy can help to detect the lesion, which includes polypoid tumor or fungating mass protruding into the lumen with ulcerated or smooth surface.
- 14. MACROSCOPIC FINDINGS Always well circumscribed and no capsule formation, while some can show ill-defined border or infiltrative growth. Solid or soft polypoid lesion locating in the mucosa or submucosa. The neoplasm also can be a round or ovoid mass which originates from any layer and even invades the wall. The cut surface color varies from grayish-white, dark red, or pinkish-grey with a central ulcer, hemorrhage and necrosis.
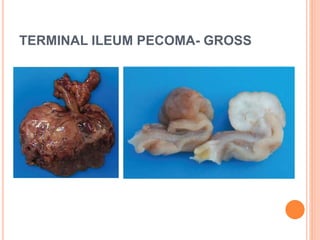
- 15. TERMINAL ILEUM PECOMA- GROSS
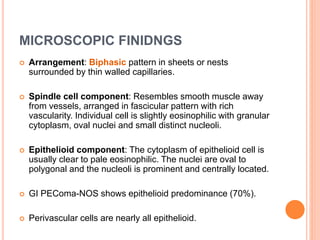
- 16. MICROSCOPIC FINIDNGS Arrangement: Biphasic pattern in sheets or nests surrounded by thin walled capillaries. Spindle cell component: Resembles smooth muscle away from vessels, arranged in fascicular pattern with rich vascularity. Individual cell is slightly eosinophilic with granular cytoplasm, oval nuclei and small distinct nucleoli. Epithelioid component: The cytoplasm of epithelioid cell is usually clear to pale eosinophilic. The nuclei are oval to polygonal and the nucleoli is prominent and centrally located. GI PEComa-NOS shows epithelioid predominance (70%). Perivascular cells are nearly all epithelioid.
- 17. SCELROSING PEComa: About 15% of cells are present in cords in a densely collagenous stroma. Focal association with blood vessel wall with radial arrangement of tumor cells and subendothelial growth is a typical feature. Typical Pecomas may contain occasional multinucleate cells, show limited pleomorphism and very scarce mitotic activity.
- 18. Microscopic features of the ileum tumor: The tumor consisted of an epithelioid cell proliferation with a vaguely nested pattern (A). In some areas, the tumor displayed a pseudoglandular histological appearance (B). The perivascular epithelioid cells had clear to eosinophilic granular cytoplasm with some slightly irregular nucleus nuclei (C). Foci of coagulation necrosis were also found in the tumor (D).
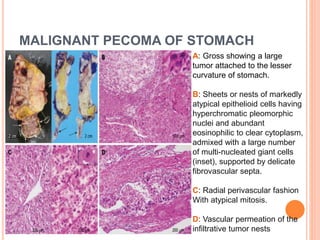
- 19. MALIGNANT PECOMA OF STOMACH A: Gross showing a large tumor attached to the lesser curvature of stomach. B: Sheets or nests of markedly atypical epithelioid cells having hyperchromatic pleomorphic nuclei and abundant eosinophilic to clear cytoplasm, admixed with a large number of multi-nucleated giant cells (inset), supported by delicate fibrovascular septa. C: Radial perivascular fashion With atypical mitosis. D: Vascular permeation of the infiltrative tumor nests
- 20. ELECTRON MICROSCOPY Cells contain abundant glycogen, melanosomes, premalanosomes, thin filaments and occasional dense bodies, hemidesmosomes and poorly formed intercellular junctions.
- 21. MALIGNANT TRANSFORMATION The 2002 WHO Soft Tissue and Bone book wrote: PEComas displaying any combination of Infiltrative growth Marked hypercellularity Nuclear enlargement Hyperchromasia High mitotic activity Atypical mitotic figures Coagulative necrosis should be regarded as malignant.
- 22. CLASSIFICATION OF PECOMA- NOS
- 23. IMMUNOHISTOCHEMISTRY HMB-45: Most sensitive Melan-A: Second most sensitive TFE3 : Potential role in the acquisition of the distinct histopathologic appearance MiTF SMA S100 Desmin CD117 CD34 Cytokeratin
- 25. FISH, PCR AND DNA SEQUENCING Rearrangement of TFE3 Gene fusions: 1. SFPQ/ PSF-TFE3 2. PSF-TFE3 3. PRCC-TFE3 4. EWS-ATF1 Up regulation of MiTF, TYR, CDK2, TBX2, and C- MET.
- 26. TFE3 GENE TFE3 gene encodes a transcription factor • TFE3 gene, located on Xp11.2, is translocated with a number of partner genes, commonly in MiT family. • Translocation often leads to overactivation of TFE3 and subsequent cell proliferation and oncogenesis. • Translocation seen in 1. Renal cell carcinoma 2. Alveolar soft part sarcoma 3. Epithelioid hemangioendotheliomas 4. Perivascular epitheloid cell tumors (PEComas) 5. Solid pseudopapillary neoplasms of the pancreas 6. Ovarian sclerosing stromal tumor
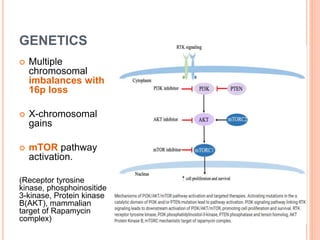
- 27. GENETICS Multiple chromosomal imbalances with 16p loss X-chromosomal gains mTOR pathway activation. (Receptor tyrosine kinase, phosphoinositide 3-kinase, Protein kinase B(AKT), mammalian target of Rapamycin complex)
- 28. PROGNOSIS PEComas with marked nuclear atypia, diffuse pleomorphism, mitotic activity (>1 mitosis/mm2) have strongest risk factor for malignancy. Borderline tumors need regular follow-up.
- 29. DIFFERENTIAL DIAGNOSIS 1. GIST PEComas and GIST show eosinophilic or clear cytoplasm but PEComa is different form GIST for its distinct perivascular concentric proliferation and representative granular cytoplasm. HMB-45 and Melan A are the most helpful tool for PEComas.
- 30. 2. MALIGNANT MELANOMA: Cells in malignant melanoma are various in shape such as epithelioid, spindle, small round, signet ring or plasmacytoid. The clinical history of primary tumor of skin is regarded as the most basic element for differential diagnosis. S100 is positive in myeloma and negative in PEComa.
- 31. 3. SMOOTH MUSCLE TUMOR: Smooth muscle tumor shows disseminate cytoplasmic eosinophilia, vacuoles around the nucleus, and “cigar- shaped” nuclei while PEComa manifests clear to lightly eosinophilic cytoplasm, and round or ovoid nuclei. HMB-45 and Melan-A are negative in smooth muscle tumor. Multinucleated giant cells promote diagnosis of PEComas
- 32. 4. RENAL CELL CARCINOMA: Chromophobe cell type has epithelioid appearance and rich angiogenesis. History of primary renal tumor is straightforward. The positive for CK, CD117, CD10, RCC antigen
- 33. 5. PARAGANGLIOMA: Has organoid growth pattern Has diffuse synaptophysin and chromogranin positivity marker but negative for myoid and melanocytic markers.
- 34. 6. GI CLEAR CELL SARCOMA Always has nests of round or epithelioid cells and mixed osteoclast-like giant cells. It expresses S100 strongly.
- 35. 7. CARCINOMA (adrenocortical or hepatocellular carcinoma): The expression of melanocytic markers (HMB-45 and Melan-A) Lack of immunoreactivity for cytokeratins help to exclude the diagnosis of carcinoma
- 36. MANAGEMENT AND FOLLOW UP Surgical resection is the most important and common method for primary tumor or local recurrence of GI PEComas. Chemotherapy and imunotherapy with IFN is given in malignant cases. The longest period of follow-up in the articles was 180 months. The patient who had radical excision with a favorable outcome Physical examination and CT scans every 6 months and yearly endoscope was also recommended.
- 37. OTHER ORGANS INVOLVED IN PECOMA- UTERUS
- 38. OTHER ORGANS INVOLVED IN PECOMA- LUNG Clear cell PEComa: (A) Core needle biopsy shows neoplastic cells in nested and insular pattern surrounding numerous thinned wall sinusoid-like vessels (B) intranuclear cytoplasmic invaginations (C) HMB45+ staining
- 39. OTHER ORGANS INVOLVED IN PECOMA- LUNG Lymphangioleiomyomatosis: • Thin walled cystic air spaces and patchy, disordered, clustered to nodular proliferation of bland spindled and cuboidal epithelioid cells around airways, lymphatics, blood vessels •Spindle cells are located centrally with peripheral epithelioid cells
- 40. OTHER ORGANS INVOLVED IN PECOMA- KIDNEY Epithelioid angiomyolipoma
- 41. OTHER ORGANS INVOLVED IN PECOMA- LIVER
- 42. REFRENCES Doyle LA, Hornick JL, Fletcher CD. PEComa of the gastrointestinal tract: clinicopathologic study of 35 cases with evaluation of prognostic parameters. Am J Surg Pathol. 2013 Dec;37(12):1769-82. Lu B, Wang C, Zhang J, et al. Perivascular epithelioid cell tumor of gastrointestinal tract: case report and review of the literature. Medicine (Baltimore). 2015;94(3):e393. Rosai, J., Ackerman, L. V., & Rosai, J. (2011). Rosai and Ackerman's surgical pathology. WHO Classification of Tumours of Soft Tissue and Bone, WHO Classification of Tumours, 4th Edition, Volume 5, Edited by Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020 Jan;76(2):182-188.
- 43. Yamada, Sohsuke & Nabeshima, Atsunori & H, Noguchi & Nawata, Aya & H, Nishii & Guo, Xin & KY, Wang & Hisaoka, Masanori & T., Nakayama. (2015). Coincidence between malignant perivascular epithelioid cell tumor arising in the gastric serosa and lung adenocarcinoma.. World Journal of Gastroenterology. 21. 1349-56.