Pharmaceutical Quality Management System
- 1. PREPARED BY- DHAWAL RAJDEV M.PHARM (QA) RK University 1
- 2. Basics of quality management system 2
- 3. Basics of quality management system Quality word oriented from Latin word ‘Qualitus’ it means General excellence OR distinctive feature. Quality- a standard of how good something is as measured against other similar things.( by OXFORD dictionary) If we try to analyse definition some common words like... 3
- 4. 1. standard 2. Measurement 3. Goodness 4. comparison Most simple definition of quality is ‘fitness for use’ The customer or user is at focous. if customer or user is happy and satisfied with our product or service than product or service is called good quality. 4
- 5. It must be remember that the quality is not the job of only a single person or single department but, it is responsibility of whole organization. Quality of Product and service is measurable, managerial, technological and stastical feature of organization. There are so many quality gurus who are first get aware about need if quality and started the evaluation of quality management at industrial level. some of them are as follows.... 5
- 6. 1.Dr. Joseph Juran He belives that quality there are no shortcut of quality, it is neither an accidental not it happens overnight. Quality must be planned. Aspects of Dr. Joseph Juran: 1. Quality planning 2. Quality control 3. Quality improvement 6
- 7. 2.Dr. W. Edward Deming He is well known for the work that he carried out in Japan in the field of quality. 1. Adopt anew policy a transformation of the style of management 2. Create purpose of continuous improvement in product and services 3. Morden methods for training the employs 4. Breakdown barriers between department 5. Create a structure of organization which allows every one to work towards transformation 7
- 8. 3.Philip B. Crosby 1. The definition of quality is to conformation requirements. 2. The system to be used is preventive of errors 3. The performance standard is zero defect 4. Measurement is based on cost of quality. 8
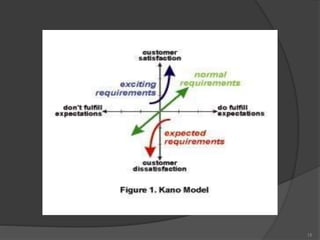
- 9. Dimensions of Quality Performance Feature Reliability Perceived quality Aesthetics Durability Conformance serviceability 9
- 11. Introduction „Total –Made up of the whole(or) Complete. „Quality –Degree of Excellence a product or service provides to the customer in present and future. „Management –Act , art, or manner of handling , controlling, directing, etc. TQM is the art of managing the whole to achieve excellence 11
- 12. "TQM is a management approach for an organization, centered on quality, based on the participation of all its members and aiming at long-term success through customer satisfaction, and benefits to all members of the organization and to society." Definition: Total Quality Management(TQM) is a management strategy aimed at embedding awareness of quality in all organizational processes. 12
- 13. TQM requires that the company maintain this quality standard in all aspects of its business. This requires ensuring that things are done right the first time and that defects and waste are eliminated from operations. Characteristics : • Technological Strength, hardness, surface finish • Time – oriented Reliability, maintainability - availability • Contractual Guarantee. Provision 13
- 15. 15
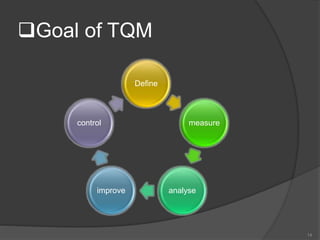
- 16. Six basic concepts of TQM 1. A committed and involved management to provide long term top – to – bottom organization support. 2. An unwavering focus on the customer, both internally and externally. 3. Effective involvement and utilization of the entire work force. 4. Continuous improvement of the business and production 16
- 17. 5. Treating suppliers of the business and production process. 6. Establishing the performance measures 17
- 19. Total Quality Management and Continuous Improvement TQM is the management process used to make continuous improvements to all functions. TQM represents an ongoing, continuous commitment to improvement. The foundation of total quality is a management philosophy that supports meeting customer requirements through continuous improvement. 19
- 20. Continuous Improvement versus Traditional Approach Traditional Approach 1. Market share focus 2. Individuals 3. Focus on who and why 4. Short term focus 5. Product focus 6. Innovation 7. fire fighting Continuous Improvement 1. Customer focus 2. Cross functional terms 3. Focus on what and how 4. Long term focus 5. Process improvement 6. Incremental improvement 7. Problem solving 20
- 21. Quality Cost Prevention Cost –Planning, Document, Control, Training Appraisal cost- inspection test installation Calibration, M/c Depreciation, Reports & Rejects. Internal Failure Cost – Scraps, Repair Rework, Design Changes, Defect Failure Analysis, Retests & Re-Inspection, Downgrading, Down Time. External Failure Cost – Complaints, Goodwill, Failures, Services & Replacement, Guarantee & Warranty, Compensation, Recall, Loss of Sales, Seconds Sales. 21
- 22. Benefits of quality Improved quality Employee participation Less migration of employee Team work Internal external customer satisfaction Productivity with connectivity Profibility & increase market share 22
- 23. 23
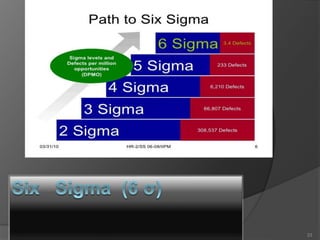
- 24. Six sigma is a business statistical Strategy. Is to identifying defects and removing them from the process of products to improve quality. A defect is defined as any process output that does not meet customer specifications. Statistical measure to objectively evaluate processes. 24
- 25. The Six sigma was founded by Motorola in the 1970s. Out of senior executive Art Sundry's criticism of Motorola’s bad quality. They founded a connection between increases in quality and decreases in costs of production. Bill Smith, “Father of six sigma” introduce this quality improvement Methodology to Motorola. 25
- 26. • Quality management program developed by Motorola in the 1980s. • Management philosophy focused on business process improvements to: Eliminate waste, rework, and mistakes Increase customer satisfaction Increase profitability and competitiveness 26
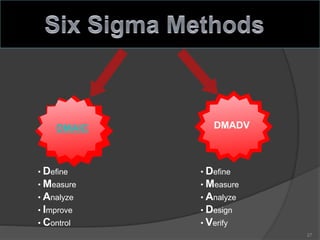
- 27. DMAIC DMADV • Define • Measure • Analyze • Improve • Control • Define • Measure • Analyze • Design • Verify 27
- 28. Define : company must identify the customer and which type of a product and hope from it. These are analyze by using flow cause/effect diagrams, check sheets, pareto analysis. Measure : company will collect the baseline data to determine where the process stands as compare to where it needs to be. And also see the critical to quality characteristics an estimate current process capability. Then find out the current sigma level according to those identified characteristic that are mostly important to the customer 28
- 29. DMAIV cont…. Analyze : this shows the amount of improvement necessary to make the Critical to quality characteristics the best in the industry. For this phase company use some descriptive statistical methods like mean, mode, median…etc. Improve : Implement the suggested improvements in this phase And also test possible solutions to the process problem. Collect data from the all possible solutions and test them on a small scale and run a cost/benefit analysis of implementing the solution. Then choose the best solution and create a plan for implement the solution. 29
- 30. 30 Improvement cycle • PDCA cycle Plan Do Chec k Act
- 31. DMAIV cont…. Control : measures are implemented to ensure improvements are maintained. To monitor the process improvements, basically use tools like statistically process control charts. These charts have three limits, the center line for the average. Monitor the process to ensure that the process is in the control limits. 31
- 32. This method is also called DFSS (Design For Six Sigma) And have five phases, Define design goals that are consistent with customer demands and the enterprise strategy. Measure and identify CTQs (characteristics that are Critical To Quality), product capabilities, production process capability, and risks. Analyze to develop and design alternatives, create a high-level design and evaluate design capability to select the best design. 32
- 33. Design details, optimize the design, and plan for design verification. This phase may require simulations. Verify the design, set up pilot runs, implement the production process and hand it over to the process owner(s). DMADV cont…. 33
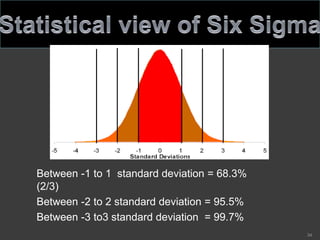
- 34. Between -1 to 1 standard deviation = 68.3% (2/3) Between -2 to 2 standard deviation = 95.5% Between -3 to3 standard deviation = 99.7% 34
- 35. •Executive Leadership (CEO and other top level managers) •Champions (act as the leaders of black belts. And also ) •Master Black Belts (chosen by champions, give their full effort to six sigma. Help to champions and guide the Black belts and green belts). •Black belts (working under Master Black Belts, they are applying six sigma to specific projects). •Green Belts (Working under the black belts). 35
- 37. 37
- 38. Focus of Six Sigma • Accelerating fast breakthrough performance • Significant financial results in 4-8 months • Ensuring Six Sigma is an extension of the Corporate culture, not the program of the month • Results first, then culture change! 38
- 39. • There is nothing new. It only proves defects and defectives counts offer tangible, measurable results. •It is corrective action system rather than taking a preventive and proactive approach to problems. •It is merely about appraisal system and that appraisal programs aren’t useful. In realty, appraisals are great tools for identifying and tracking improvements, which is critical to any project. •Critics have suggested that Six Sigma did not bring quality improvement in all the organizations where it was implemented. It depends on the tools and authorizations. 39
- 40. Management philosophy of quality Components of Six Sigma are people power and process power Define, Measure, Analyze, Improve, Control Criticisms Executive Leader, Champion, Master Black Belt, Black Belt, and Green Belt Statistical target of six sigma or 3.4 defects in one million opportunities Summary 40
- 41. 41
- 42. International Organisation for standardization (ISO) A network of national standardization bodies from over 160 countries with Nigeria inclusive. Based in Geneva Switzerland. Standard Organisation of Nigeria (SON) is a Technical Committee (TC) in ISO, meaning participates fully in developing ISO standards. 42
- 43. ISO management standards Selected standards that companies can be certified for. ISO 9001 Quality ISO 22000 Food safety ISO 22301 Business continuity ISO 20000 IT services ISO 14001 Environment OHSAS 18001 Health and Safety ISO 28000 Supply chain Security ISO 27001 Information security 43
- 44. ISO 9001:2008 (QMS)basic principles Principle 1 – Customer focus Principle 2 – Leadership Principle 3 – Involvement of people Principle 4 – Process approach Principle 5 – System approach to management Principle 6 – Continual improvement Principle 7 – Factual approach to decision making Principle 8 – Mutually beneficial supplier relationships It is upon these principles that an organization can imbibe a sound quality system. 44
- 45. Principle 1 - Customer focus Pragmatically, a quality product centers on meeting customers current and future requirements in the needed form, time and place in synchrony with the company’s policies and objectives. Customer satisfaction Employee’s Management responsibility responsibility Customer requirements QMS 45
- 46. Application and Benefits of Principle 1 what needs to be done Management must be committed. Consistent research into customers needs and expectations. Measuring customers satisfaction with prompt action taken. Company’s objectives and policies must be linked with customers requirements and interest of other parties(financers, local communities). All employees must be aware of customers needs and expectations. Strategically managing customers relationship. what will happen? Increased market share. Efficient and effective use of company’s recourses geared towards customers satisfaction. Increased customer trust and loyalty 46
- 47. Principle 2 - Leadership Application Perfect understanding of the organization short and long term goals and objectives. Setting realistic and practicable targets. Make the needs of all stakeholders a focal point. Maintain a trustful work ambience and not a fearful environment. Positive contributions must be encouraged. Benefits More enthusiastic workers. Increased employee loyalty Effective communication system is enabled. 47
- 48. Principle 3 - Involvement of the peopleApplications A sense of belonging should be created in people that has direct and indirect link with the company. People should be made to understand that their opinion counts. People openly discussing problems and issues. People identifying constraints to their performance. Benefits Employees shows more commitment towards achieving organization goals and objectives. Innovation and creativity within the organization. People show more interest in continuous improvement. 48
- 49. Principle 4 - Process Approach Input Output Customers (and other interested parties) Customers (and other interested parties) Management responsibility Resource management Product realization Measurement, analysis and improvement Requirement s Satisfaction Continual improvement of the quality management system. 49
- 50. What do we need to do? Highlighting the activities necessary to obtain a desired output Establishing clear responsibility and accountability for managing key activities Analyzing and measuring of the capability of key activities Identifying the interfaces of key activities within and between the functions of the organization Focusing on the factors such as resources, methods, and materials that will improve key activities of the organization Evaluating risks, consequences and impacts of activities on customers, suppliers and other interested parties. Consequences Effective usage of available recourses Cost of production is reduced due to clear definition of activities Focus is given to opportunities that will cause continuous improvement in the system. 50
- 51. Principle 5 - System Approach to Management System = combinations of entities (processes)that works dependently and interrelated with each other and becomes a culture over a period of time. Management responsibility Measurement, analysis and improvement Product realization Resource management System 51
- 52. Requirements Conceiving a structured system that meets organization objectives efficiently and effectively. Understanding and defining the interrelationships and interdependencies between each processes within the system. Devising ways to harmonise and integrating the related processes. Providing clear definitions of individual roles and responsibilities towards achieving a common objectives to prevent cross- functional barriers. Reconciling the organization targets with the resources available to know possible limits so as to fashion a way out. Targeting and defining how specific activities within a system should operate. Continually improving the system through measurement and evaluation. What will happen? Integration and alignment of the processes that will best achieve the desired results Ability to focus effort on the key processes Providing confidence to interested parties as to the consistency, effectiveness and efficiency of the organization. 52
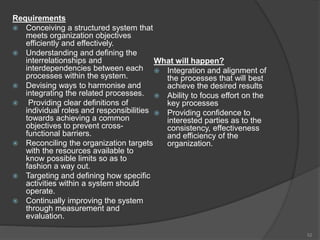
- 53. Principle 6 - Continual ImprovementHow do we achieve this? Frequent internal audit of the quality system identifying weak spots and areas that needs improvement. Employing a consistent organization-wide approach to continual improvement of the organization’s performance Providing people with training in the methods and tools of continual improvement Making continual improvement of products, processes and systems an objective for every individual in the organization Establishing goals to guide, and measures to track, continual improvement Recognizing and acknowledge and improvements. Benefits Performance advantage through improved organizational capabilities Alignment of improvement activities at all levels to an organization’s strategic intent Flexibility to react quickly to opportunities. 53
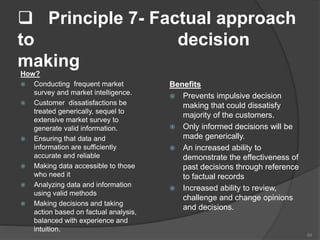
- 54. Principle 7- Factual approach to decision making How? Conducting frequent market survey and market intelligence. Customer dissatisfactions be treated generically, sequel to extensive market survey to generate valid information. Ensuring that data and information are sufficiently accurate and reliable Making data accessible to those who need it Analyzing data and information using valid methods Making decisions and taking action based on factual analysis, balanced with experience and intuition. Benefits Prevents impulsive decision making that could dissatisfy majority of the customers. Only informed decisions will be made generically. An increased ability to demonstrate the effectiveness of past decisions through reference to factual records Increased ability to review, challenge and change opinions and decisions. 54
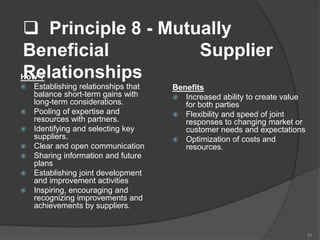
- 55. Principle 8 - Mutually Beneficial Supplier RelationshipsHow ? Establishing relationships that balance short-term gains with long-term considerations. Pooling of expertise and resources with partners. Identifying and selecting key suppliers. Clear and open communication Sharing information and future plans Establishing joint development and improvement activities Inspiring, encouraging and recognizing improvements and achievements by suppliers. Benefits Increased ability to create value for both parties Flexibility and speed of joint responses to changing market or customer needs and expectations Optimization of costs and resources. 55
- 56. Conclusion All discussed principles are the core attributes on which ISO 9001:2008 are based upon, and it is therefore imperative that to meet up with International standards, we at ACSK should strive to align our operations with these principles to foster a better competitive advantage both on local and international front. 56
- 57. Recommendations 1. GAP analysis of the status quo. Current state Desired / future state of the company 2. Assembling a Quality Management Team (QMT)with following responsibilities: Performing timely frequent internal audit of the quality management status of the company. Establishing a corrective and preventive action plan for the diagnosed nonconformities with ISO 9001. Piloting and Charting the course and directions towards the company certification for ISO 9001. 3. Research and Development (R&D)department be considered as a long-term competitive strategy. GAP 57
- 58. 58
- 59. Are you ready for changes? Prepared by: Reza Seifollahy Background First published in 1987, ISO 9000 has consistently been ISO’s most popular series of standards. building on 25 years of success, ISO technical committee ISO/TC 176,Quality management and quality assurance, subcommittee SC 2, Quality systems, is busy laying the groundwork for the next generation of quality management standards. 59
- 60. Are you ready for Changes? Prepared By: Reza Seifollahy ISO 9001 revisions since beginning 1987 1994 2000 2008 2015 Revisions 60
- 61. ISO 9000 Series Prepared by: Reza Seifollahy They have: Consistently been ISO’s best-selling standards; Firmly established a common platform and language for organizations to discuss quality; By defining requirements in ISO 9001, Quality management systems, that give a base-level confidence in an organization’s ability to provide conforming products, they have facilitated world trade. Are you ready for changes? 61
- 62. Are you ready for changes?Latest ISO 9000 series ISO9001:2008 is Quality management systems – Requirements Previous Add latest Versions Prepared by: Reza Seifollahy ISO9004:2009 is Managing for the sustained successof an organization – A quality management approach 62
- 63. Looking to the Future s Are you ready for the Changes? Prepared by: Reza Seifollahy The vision of standard preparation committee is looking a head to the needs of future. 63
- 64. Basic Principles are revisited from eight to seven REVISION OF QUALITY PRINCIPLES Are you ready for changes? Prepared by: Reza Seifollahy 64
- 65. 4. Process Approach Quality Principles The Revised Quality Principles Are you ready for the changes? 65
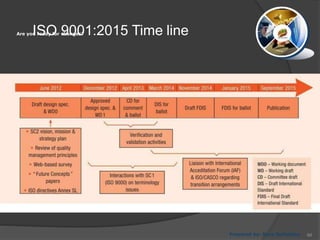
- 66. ISO 9001:2015 Time lineAre you ready for changes? Prepared by: Reza Seifollahy 66
- 67. 10 Clues 1. Scope 2. Normative references 3. Terms and definition 4. Context of organization 5. Leadership 6. Planning 7. Support 8. Operations 9. Performance Evaluation 10. Improvement 67
- 68. Proposed Clauses: Prepared by: Reza Seifollahy 1. Scope 2. Normative references 3. Terms and Definitions 1. Organization 2. Interested Party (preferred term) 3. Requirement 4. Management System 5. Top management 6. Effectiveness 7. Policy Are you ready for the changes? 68
- 69. Proposed Clauses: Prepared by: Reza Seifollahy 8. Objective 9. Risk 10. Competence 11. Documented information 12. Process 13. Performance 14. Outsource (verb) 15. Monitoring 16. Measurement process 17. Audit Are you ready for the changes? 69
- 70. Proposed Clauses: Prepared by: Reza Seifollahy 18. Conformity 19. Non-conformity 20. Correction 21. Corrective Action 22. Continual improvement Are you ready for the changes? 70
- 71. 4. Context of The Organization Prepared by: Reza Seifollahy 1. Understanding the Organization and its context 2. Understanding the needs and expectations of interested parties 3. Determining the scope of the quality management system 4. Quality Management System 1. General 2. Process Approach Are you ready for the changes? 71
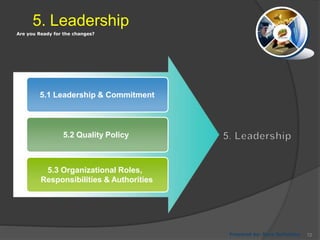
- 72. Are you Ready for the changes? 5. Leadership 5.1 Leadership & Commitment 5.2 Quality Policy 5.3 Organizational Roles, Responsibilities & Authorities Prepared by: Reza Seifollahy 72
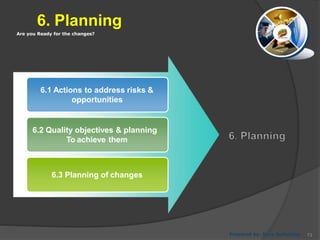
- 73. Are you Ready for the changes? 6. Planning 6.1 Actions to address risks & opportunities 6.2 Quality objectives & planning To achieve them 6.3 Planning of changes Prepared by: Reza Seifollahy 73
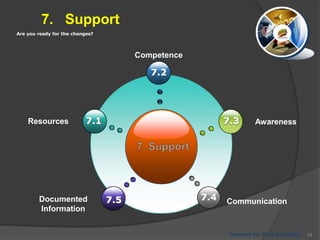
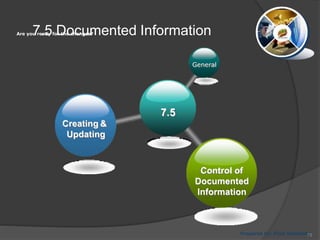
- 74. Are you ready for the changes? 7. Support 7.5 7.3 7.4 7.1Resources Prepared by: Reza Seifollahy Competence 7.2 Awareness Documented Information Communication 74
- 75. Are you ready for the changes? Prepared by: Reza Seifollahy 7.5 Documented Information 7.5 General Creating & Updating Control of Documented Information 75
- 76. 8. Operation Prepared by: Reza Seifollahy 1.Operational Planning & Control 2.Determination of Market needs & interactions with customers 3.Operational Planning process 4.Control of external Provision of Goods & Services 5.Development of Goods & Services 6.Production of goods and provision of services 7.Release of goods and services 8.Nonconforming goods & services Are you ready for the changes 76
- 77. Prepared by: Reza Seifollahy The new clause 8 is covered the requirements of clause 7 in last version. Clause 7.3 design & development in previous versions is simplified to clause 8.5 development of goods and services. The new standard resolved many problems related to design and development. Are you ready for the changes? 77
- 78. 9. Performance evaluation 9.1. 9.2. 9.3. Monitoring, measurement , analysis and evaluation 9.1.1.General, 9.1.2.customer satisfaction, 9.1.3. Analysis & evaluation of data Internal Audit Management Review Prepared by: Reza Seifollahy Are you ready for the changes? 78
- 79. 10.1 Nonconformity and Corrective Action 10.2 Improvement 10. Continual Improvement Are you ready for the changes? 79
- 80. Management Service Division ISO 14001:2004 Overview 80
- 81. It was due for revision, ISO requires that all management system standards undergo periodic revision To enhance compatibility with ISO 9001:2000 Revision allows opportunity to clarify requirements in the 1996 version and incorporate needed changes Why was ISO 14001 revised? 81
- 82. 4.1 General Requirements The organization shall establish, document, implement, maintain and continually improve an environmental management system in accordance with the requirements of this International Standard and determine how it will fulfill these requirements. The organization shall define and document the scope of its environmental management system. ISO 14001:2004 82
- 83. Environmental Policy ISO 14001: 2004 Top management shall define the organization's environmental policy and ensure that, within the defined scope of its environmental management system, it: a) is appropriate to the nature, scale and environmental impacts of its activities, products and services, b) includes a commitment to continual improvement and prevention of pollution, 83
- 84. ISO 14001: 2004 c) includes a commitment to comply with applicable legal requirements and with other requirements to which the organization subscribes which relate to its environmental aspects, d)provides the framework for setting and reviewing environmental objectives and targets, 84
- 85. ISO 14001: 2004 e)is documented, implemented and maintained f) is communicated to all persons working for or on behalf of the organization, and g)is available to the public. 85
- 86. ISO 14001: 2004 The organization shall establish, implement and maintain a procedure(s) a)to identify the environmental aspects of its activities, products and services within the defined scope of the environmental management system that it can control and those that it can influence taking into account planned or new developments, or new or modified activities, products and services, and 86
- 87. ISO 14001: 2004 b)to determine those aspects that have or can have significant impact(s) on the environment (i.e. significant environmental aspects). The organization shall document this information and keep it up to date. The organization shall ensure that the significant environmental aspects are taken into account in establishing, implementing and maintaining its environmental management system. 87
- 88. Objectives, targets and programme(s) The organization shall establish, implement and maintain documented environmental objectives and targets, at relevant functions and levels within the organization. The objectives and targets shall be measurable, where practicable, and consistent with the environmental policy, including the commitments to prevention of pollution, to compliance with applicable legal requirements and with other requirements to which the organization subscribes, and to continual improvement. 88
- 89. ISO 14001: 2004 b)to determine those aspects that have or can have significant impact(s) on the environment (i.e. significant environmental aspects). The organization shall document this information and keep it up to date. The organization shall ensure that the significant environmental aspects are taken into account in establishing, implementing and maintaining its environmental management system. 89
- 90. When establishing and reviewing its objectives and targets, an organization shall take into account the legal requirements and other requirements to which the organization subscribes, and its significant environmental aspects. It shall also consider its technological options, its financial, operational and business requirements, and the views of interested parties. 90
- 91. ISO 14001: 2004 b)to determine those aspects that have or can have significant impact(s) on the environment (i.e. significant environmental aspects). The organization shall document this information and keep it up to date. The organization shall ensure that the significant environmental aspects are taken into account in establishing, implementing and maintaining its environmental management system. 91
- 92. ISO 14001: 2004 b)to determine those aspects that have or can have significant impact(s) on the environment (i.e. significant environmental aspects). The organization shall document this information and keep it up to date. The organization shall ensure that the significant environmental aspects are taken into account in establishing, implementing and maintaining its environmental management system. 92
- 93. ISO 14001: 2004 b)to determine those aspects that have or can have significant impact(s) on the environment (i.e. significant environmental aspects). The organization shall document this information and keep it up to date. The organization shall ensure that the significant environmental aspects are taken into account in establishing, implementing and maintaining its environmental management system. 93
- 94. ISO 14001: 2004 b)to determine those aspects that have or can have significant impact(s) on the environment (i.e. significant environmental aspects). The organization shall document this information and keep it up to date. The organization shall ensure that the significant environmental aspects are taken into account in establishing, implementing and maintaining its environmental management system. 94
- 95. Resources, roles, responsibility and authority Management shall ensure the availability of resources essential to establish, implement, maintain and improve the environmental management system. Resources include human resources and specialized skills, organizational infrastructure, technology and financial resources. Roles, responsibilities and authorities shall be defined, documented and communicated in order to facilitate effective environmental management. 95
- 96. ISO 14001: 2004 b)to determine those aspects that have or can have significant impact(s) on the environment (i.e. significant environmental aspects). The organization shall document this information and keep it up to date. The organization shall ensure that the significant environmental aspects are taken into account in establishing, implementing and maintaining its environmental management system. 96
- 97. The organization's top management shall appoint a specific management representative(s) who, irrespective of other responsibilities, shall have defined roles, responsibilities and authority for a)ensuring that an environmental management system is established, implemented and maintained in accordance with the requirements of this International Standard, 97
- 98. reporting to top management on the performance of the environmental management system for review, including recommendations for improvement. 98
- 99. Communication With regard to its environmental aspects and environmental management system, the organization shall establish, implement and maintain a procedure(s) for a) internal communication among the various levels and functions of the organization, b) receiving, documenting and responding to relevant communication from external interested parties. 99
- 100. The organization shall decide whether to communicate externally about its significant environmental aspects, and shall document its decision. If the decision is to communicate, the organization shall establish and implement a method(s) for this external communication 100
- 101. Documentation The environmental management system documentation shall include a) the environmental policy, objectives and targets, b) description of the scope of the environmental management system, c) description of the main elements of the environmental management system and their interaction, and reference to related documents, d) documents, including records, required by this International Standard, and e) documents, including records, determined by the organization to be necessary to ensure the effective planning, operation and control of processes that relate to its significant environmental aspects. 101
- 102. Internal audit Audit programme(s) shall be planned, established, implemented and maintained by the organization, taking into consideration the environmental importance of the operation(s) concerned and the results of previous audits. Audit procedure(s) shall be established, implemented and maintained that address the responsibilities and requirements for planning and conducting audits, reporting results and retaining associated records, — the determination of audit criteria, scope, frequency and methods. Selection of auditors and conduct of audits shall ensure objectivity and the impartiality of the audit process. 102
- 103. Pharmaceutical Quality Management ICH Q-10 103
- 104. ICH Q-10 ICH Q10 demonstrates industry and regulatory authorities’ support of an effective pharmaceutical quality system to enhance the quality and availability of medicines around the world in the interest of public health. For the purposes of this guidance, the product lifecycle includes the following technical activities for new and existing products: 104
- 105. ICH Q10 provides a harmonized model for a pharmaceutical quality system throughout the lifecycle of a product and is intended to be used together with regional GMP requirements 105
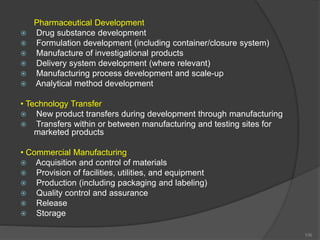
- 106. Pharmaceutical Development Drug substance development Formulation development (including container/closure system) Manufacture of investigational products Delivery system development (where relevant) Manufacturing process development and scale-up Analytical method development • Technology Transfer New product transfers during development through manufacturing Transfers within or between manufacturing and testing sites for marketed products • Commercial Manufacturing Acquisition and control of materials Provision of facilities, utilities, and equipment Production (including packaging and labeling) Quality control and assurance Release Storage 106
- 107. ICH Objectives Implementation of the Q10 model should result in achievement of three main objectives that complement or enhance regional GMP requirements. Achieve Product Realization To establish, implement, and maintain a system that allows the delivery of products with the quality attributes appropriate to meet the needs of patients, health care professionals, regulatory authorities (including compliance with approved regulatory filings) and other internal and external customers 107
- 108. Establish and Maintain a State of Control To develop and use effective monitoring and control systems for process performance and product quality, thereby providing assurance of continued suitability and capability of processes. Quality risk management can be useful in identifying the monitoring and control systems Facilitate Continual Improvement To identify and implement appropriate product quality improvements, process improvements, variability reduction, innovations, and pharmaceutical quality system enhancements, thereby increasing the ability to fulfill a pharmaceutical manufacturer’s own quality needs consistently. Quality risk management can be useful for identifying and prioritizing areas for continual improvement 108
- 109. Quality Risk Management Quality risk management is integral to an effective pharmaceutical quality system. It can provide a proactive approach to identifying, scientifically evaluating, and controlling potential risks to quality. It facilitates continual improvement of process performance and product quality throughout the product lifecycle. ICH Q9 provides principles and examples of tools for quality risk management that can be applied to different aspects of pharmaceutical quality. 109
- 110. Design and Content Considerations The design, organization, and documentation of the pharmaceutical quality system should be well structured and clear to facilitate common understanding and consistent application The pharmaceutical quality system should include appropriate processes, resources, and responsibilities to provide assurance of the quality of outsourced activities and purchased materials, etc. 110
- 111. The size and complexity of the company’s activities should be taken into consideration when developing a new pharmaceutical quality system or modifying an existing one. The design of the pharmaceutical quality system should incorporate appropriate risk management principles. The elements of ICH Q10 should be applied in a manner that is appropriate and proportionate to each of the product lifecycle stages, recognizing the different goals and knowledge available for each stage. 111
- 112. Quality Manual A Quality Manual or equivalent documentation approach should be established and should contain the description of the pharmaceutical quality system. The description should include: (a) The quality policy (b) The scope of the pharmaceutical quality system. (c) Identification of the pharmaceutical quality system processes, as well as their sequences, linkages, and interdependencies. Process maps and flow charts can be useful tools to facilitate depicting pharmaceutical quality system processes in a visual manner. (d) Management responsibilities within the pharmaceutical quality system 112
- 113. 113
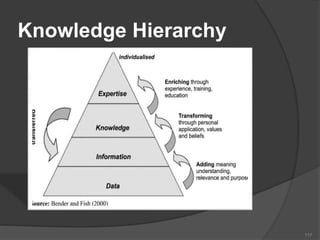
- 114. contents Data, Information & Knowledge Knowledge Hierarchy Types of Knowledge What Is Knowledge Management Why KM History of KM KM Models KM life cycle 114
- 115. Continue… Characteristics of KM in Libraries Terms Used in KM 115
- 118. Explicit/ Tacit Knowledge- Types Tacit knowledge: That type of knowledge which people carry in their mind, and is, therefore, difficult to access. Explicit knowledge: That type of knowledge which has been or can be articulated, codified, and stored in certain media. 118
- 119. Features Explicit Knowledge Tacit Knowledge Tangible Intangible Physical objects, e.g. in documents or databases Mental objects, i.e. it's in people's head's Context independent Context affects meaning Easily shared Sharing involves learning Reproducible Not identically replicated 119
- 120. What Is Knowledge Management Knowledge Management is the collection of processes that govern the creation, dissemination, and utilization of knowledge. 120
- 121. Why KM To share the knowledge, a company creates exponential benefits from the knowledge as people learn from it. To build better sensitivity to “brain drain” To reacting to new business opportunities 121
- 122. History In 70’S A number of management theorists have contributed to the evaluation of KM. Peter Drucker: Information and knowledge as organizational resources Peter Senge: "learning organization" Chaparral Steel: A company having knowledge management strategy 122
- 123. In 80’s Knowledge as a competitive asset was apparent. Managing knowledge that relied on work done in artificial intelligence and expert systems. Knowledge management-related articles began appearing in journals and books . 123
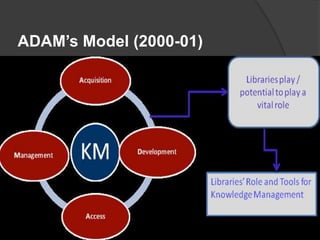
- 124. In 90’s Until Now A number of management consulting firms had begun in-house knowledge management programs. E.g. ADAM’s Model Knowledge management was introduced in the popular press. The International Knowledge Management Network(IKMN) went online in 1994. 124
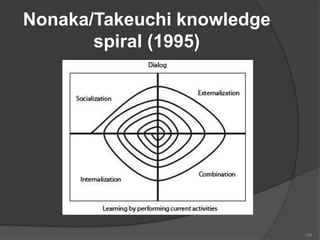
- 125. KM Models There are some KM Models: Nonaka/Takeuchi Knowledge Spiral (1995) ADAM’s Model (2000-01) The Choo Sense-making KM Model (1998) WIIG KM Model 125
- 128. The Choo Sense-making KM Model (1998) 128
- 129. WIIG’s KM Model 129
- 130. Knowledge Form by WIIG Model 130 Public Knowledge Sharing Knowledge Personal Knowledge
- 131. CONTINUE… The knowledge which is explicit and can be learned and shared, called Public Knowledge. The knowledge which is an intellectual assets and held exclusively by employees and shared during work or embedded in technologies, called Sharing Knowledge. The knowledge which is the least accessible, but the most complete form of knowledge. It’s usually tacit and used without knowing, called Personal Knowledge. 131
- 132. Knowledge Types by WIIG Model Factual Knowledge Conceptual Knowledge Expectational Knowledge Methodological Knowledge 132
- 133. Continue… That type of knowledge which deals with data and measurements, and directly observable and verifiable, called Factual Knowledge. That type of knowledge which deals with systems, concepts and perspectives, called Conceptual Knowledge. That type of knowledge which deals with hypothesis, judgments and expectations held by knowers, called Expectational Knowledge. 133
- 134. Continue… That type of knowledge which deals with reasoning, strategies and decision making methods, called Methodological Knowledge. 134
- 135. o Knowledge management life cycle 135
- 136. Continue… Information Mapping: ( To categorize the knowledge assest) Information mapping is a process by which organizations can identify and categories knowledge assets within their organization. Information Storaging: Information storing that contains knowledge repositories such as databases, data warehouses, and information centers and indicates electronic environment of organizational memory. 136
- 137. continues… Information Retrieving: In this stage, knowledge is stored and retrieved via information retrieval systems. 137
- 138. Knowledge Using Organizations use knowledge for three reasons: Knowledge can be used for determining organization’s work processes and making strategies for sustainable competitive advantage. Knowledge can be used for designing and marketing product. Knowledge plays a critical role of organization’s services quality 138
- 139. Knowledge Auditing Knowledge auditing means what amount of knowledge can be used in organization’s products, services and processes. 139
- 140. Characteristics of KM in Libraries The characteristics of KM in libraries are: Human Resource Management Is the Core of Knowledge Management in Libraries. The Objective of Knowledge Management in Libraries is to Promote Knowledge Innovation. Information Technology Is a Tool for Knowledge Management in Libraries. The knowledge acquired must be accumulated and converged into knowledge warehouses of libraries. 140
- 141. Terms Used in KM There are some terms used in KM: Knowledge architect Knowledge assets Knowledge bridge Knowledge Workers Knowledge Economy 141
- 142. Knowledge architect Knowledge architect is the staff member who oversees the definitions of knowledge and intellectual processes and then identifies the technological and human resources required to create, capture, organize, access and use knowledge assets. 142
- 143. Knowledge assets Knowledge assets, also called intellectual capital, are the human, structural and recorded resources available to the organization. Assets reside within the minds of members, customers, and colleagues and also include physical structures and recorded media. 143
- 144. Knowledge bridge Knowledge bridge is the connection that a KM expert builds between the business processes and the technological, sociological, personal, financial, sales, creative, and customer oriented functions of the organization. 144
- 145. Knowledge Workers Employees and managers who contribute significantly to the intellectual capital of the company are called knowledge workers. 145
- 146. Knowledge Economy The knowledge economy is a term that refers either to an economy of knowledge focused on the production and management of knowledge in the frame of economic constraints, or to a knowledge-based economy. 146
- 147. 147
- 148. Introduction Quality metrics are used throughout the drugs and biologics industry to monitor quality control systems and processes and drive continuous improvement efforts in drug manufacturing This is an explanation of how the Center for Drug Evaluation and Research (CDER) and the Center for Biologics Evaluation and Research (CBER) intend to utilize submitted data and quality metrics to help ensure that their policies and practices continue to support continuous improvement and innovation in the pharmaceutical manufacturing industry. 148
- 149. In order to achieve these goals, FDA is initiating a quality metrics reporting program. As described in this guidance. In the voluntary reporting phase of the program, FDA expects to learn more about a limited set of quality metrics, associated analytics, and improve the FDA quality metrics reporting program In general, FDA’s guidance documents do not establish legally enforceable responsibilities. The use of the word should is used to indicate an FDA preference to promote consistent reporting and counting of quality metrics data 149
- 150. Quality matrix that FDA intended to calculate The following set of quality metrics that FDA intends to calculate based on industry reporting was developed with stakeholder input. FDA used the following selection criteria in developing the set of data that it is inviting covered establishments to submit (1) Objective data to provide consistency in reporting, (2) of the type contained in records subject to inspection under section of the FD&C Act, and (3) a valuable component in assessing the overall effectiveness of a PQS, within reasonable limits, and in a reasonable manner, while avoiding an undue reporting burden 150
- 151. Using reported data described in the following section, FDA intends to calculate quality metrics for each product and covered establishment, where applicable: Lot Acceptance Rate (LAR) as an indicator of manufacturing process performance. LAR = the number of accepted lots in a timeframe divided by the number of lots started by the same covered establishment in the current reporting timeframe. Product Quality Complaint Rate (PQCR) as an indicator of patient or customer feedback. PQCR = the number of product quality complaints received for the product divided by the total number of dosage units distributed in the current reporting timeframe. 151
- 152. Invalidated Out-of-Specification (OOS) Rate (IOOSR) as an indicator of the operation of a laboratory. IOOSR = the number of OOS test results for lot release and long-term stability testing invalidated by the covered establishment due to an aberration of the measurement process divided by the total number of lot release and long-term stability OOS test results in the current reporting timeframe. 152
- 153. How to Report Quality matrix data in FDA Product Reporting Establishments Product Reporter Top Tier: If complete data supporting all metrics were included for each covered establishment in the manufacturing supply chain for all covered drug products (or APIs used in the manufacture of a covered drug product) for the full year reporting period Product Reporter Mid Tier: If all covered establishments in the manufacturing supply chain for all covered products were identified in the report, and complete quality metric data was provided from at least one of the establishments for each covered drug products (or APIs used in the manufacture of a covered drug product) for the full year reporting period 153
- 154. Product Supply Chain Reporter: If all covered establishments in the manufacturing supply chain for all covered drug products (or APIs used in the 504 manufacture of a covered drug product) were identified in the report 154
- 155. Site Reporting Establishments Site Reporter Top Tier: If complete data supporting all metrics were included for all covered drug products (or APIs used in the manufacture of a covered drug product) for the full year reporting period Site Reporter Mid Tier: If complete data supporting all metrics were included for at least one covered drug product (or API used in the manufacture of a covered drug product) manufactured at an establishment for the full year reporting period 155
- 156. 156
- 157. OSHAS OHSAS 18000 is an international occupational health and safety advisory service specification. Originally developed in early 1990’s as BS8800. Revised in 1999 by BSI to be more compatible with ISO 14001. It comprises two parts, 18001 and 18002 and embraces BS8800 and a number of other publications. 17 elements designed in parallel to ISO 14001 and allows third-party certification/registration. 157
- 158. An international standard like ISO 9001or ISO 14001. An international guideline as ICH. Not prescriptive –no absolute requirements; very similar to ISO 14001. OHSAS 18001 was created via a concerted effort from a number of the worlds leading national standards bodies, certification bodies, and specialist consultancies. 158
- 159. Documents used in creation of OSHAS BS8800:1996 Guide to occupational health and safety management systems DNV Standard for Certification of Occupational Health and Safety Management Systems(OHSMS):1997 Technical Report NPR 5001: 1997 Guide to an occupational health and safety management system. Draft LRQA SMS 8800 Health & safety management systems assessment criteria 159
- 160. SGS & ISMOL ISA 2000:1997 Requirements for Safety and Health Management Systems BVQI Safety Cert: Occupational Safety and Health Management Standard Draft AS/NZ 4801 Occupational health and safety management systems Specification with guidance for use Draft BSI PAS 088 Occupational health and safety management systems UNE 81900 series of pre-standards on the Prevention of occupational risks Draft NSAI SR 320 Recommendation for an Occupational Health and Safety (OH and S) Management System 160
- 161. Participants in creation of OSHASNational Standards Authority of Ireland. Standards Australia. South African Bureau of Standards. British Standards Institution. Bureau Veritas Quality International. Lloyds Register Quality Assurance. National Quality Assurance. SFS Certification. SGS Yarsley International Certification Services. Asociaci?spa? de Normalizaci? Certificaci?r . International Safety Management Organisation Ltd. Standards and Industry Research Institute of Malaysia. International Certification Services . 161
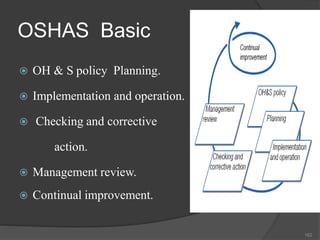
- 162. OSHAS Basic OH & S policy Planning. Implementation and operation. Checking and corrective action. Management review. Continual improvement. 162
- 163. OH & S Policy Clearly states overall OH&S objectives. Authorized by top management. Appropriate to nature & scale of OH&S risks. Documented, implemented, and maintained. Communicated to all employees. Available to interested parties. Reviewed periodically. 163
- 164. OH&S Policy Commitments •Improve health & safety performance* Continual improvement. “at least” comply with current applicable . OH&S legislation and other requirements. * Performance: measurable results of the OH&S management system, related to the organization’s control of health and safety risks, based on its OH&S policy and objectives. 164
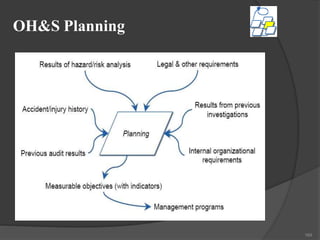
- 165. OH&S Planning Hazard identification, risk assessment, and risk control. • Legal and other requirements. Objectives OH&S management program(s) 165
- 166. Hazard Identification, Risk Assessment & Risk Control Conceptually similar to environmental aspects and impacts –target of management program(s)• Much more detailed than 14001 approach Assessment must address: – routine and non-routine activities – all personnel, including contractors and visitors – facilities at the workplace, whether provided by the organization or by others 166
- 167. Hazard Identification, Risk Assessment & Risk Control • • Methodology must be proactive – in advance of process/equipment changes – allow engineering of hazard controls during design – implementation of controls as change occurs Success requires strong Management of Change (MOC) procedure 167
- 168. Hazard Identification, Risk Assessment & Risk Control • • Process overview –identification of hazards –evaluation of risks under current controls –evaluation of the tolerability of residual risk –identification of needed additional controls People are involved –significant risks must be controlled –individual behaviour is a significant factor 168
- 170. Implementation & Operation Structure and responsibilities Training awareness and competence Consultation and communication Documentation Document and data control Operational control • • • • • • • Emergency preparedness and response 170
- 171. Structure & Responsibilities Documented roles, responsibilities, authorities, and accountability Management appointee responsible for implementation Resources Managers must demonstrate commitment to continual improvement 171
- 172. Training, Awareness & Competence Ensure employee awareness aAnd competence • Take into account differing levels of: – Responsibility – Ability – Literacy – Risk Much of required training driven by regulation 172
- 173. Consultation & Communication More internally focused than ISO 14001 Employee involvement and consultation –in development/review of policies and procedures –about changes that affect workplace safety or health –ensuring representation in OH&S matters Buy-in, ownership, motivation 173
- 174. Documentation & Data • • Documentation of core elements –aids employee awareness. –shows how the various system relate. –extremely valuable during certification process. Document and data control procedures –critical documents are available –obsolete documents and data are removed 174
- 175. Operational Control • • Identify operations and activities where risk requires further control Plan these to ensure that – documented procedures are developed – operating criteria specify key steps and requirements – procedures addressing risks related to contractor goods and services – establish design procedures to reduce/eliminate source of risks 175
- 177. Emergency Preparedness & Response Emergency response procedures to address – identifying potential for incidents and emergencies. – preventing and mitigating resultant illnesses and injuries. – responding to incidents and emergencies when they occur. 177
- 179. Checking & Corrective Action Performance measurement and monitoring. Accidents, incidents, non-conformances and corrective and preventive action. Records and records management. OH&S management system audit. 179
- 180. Performance Measurement & Monitoring • • • • • Monitoring the achievement of objectives. Quantitative and qualitative measures. Proactive and reactive methods. Records to facilitate corrective and preventive actions. Calibration of monitoring equipment. 180
- 181. Quantitative & Qualitative • • Direct Quantitative Measures –number of lost work days following an injury. –decibel levels of noise in a work area. Indirect Qualitative Measures –review of inspection logs. –observation of a task. –Interviews. 181
- 182. Proactive & Reactive Measures • • Proactive monitoring of compliance – routine basis, independent of any event. – monitoring may be required by regulations • daily equipment checks. • periodic review of hot-work permits. Reactive monitoring of accidents or incidents – in response to an event or trigger •accident investigation. •monitoring in response to a complaint. 182
- 183. Accident, Incidents, Non- conformances & Corrective and Preventive Action • • • Handle, investigate, mitigate –Accidents. –Incidents. –non-conformances. Corrective and preventive actions. Review action plans through risk assessment process. 183
- 184. Accident, Incidents & Non-conformances • • • Handle = immediate action –Notification. –emergency response. –recordkeeping to facilitate investigation. Investigation process. –team and procedures. –root cause analysis. People are involved –human elements. 184
- 185. Corrective and Preventive Action Correct immediate problem. •Mitigate consequences. Eliminate or control root cause. Prevent recurrence. Review action plans through risk assessment process. Communicate results and monitor. 185
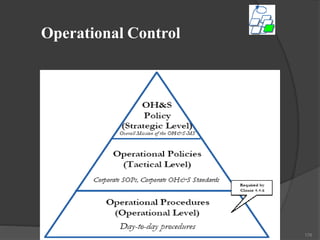
- 186. Records & Record Management • • Identification, maintenance, and disposition Records must be: – Legible. – Identifiable. – traceable to the activities involved. – easily retrievable. – protected from damage, deterioration, or loss. – held for specified and documented retention times. 186
- 187. OH&S Management System Audit • • Determine if OH&S-MS: –conforms with planned arrangements. –is properly implemented and maintained. –is effective in meeting policy and objectives. Results provided to top management. • Audit program and schedule reflect risks and previous audit results. 187
- 189. CONCLUSION Essentially, OHSAS helps in a variety of respects... it helps: minimize risk to employees/etc; improve an existing OH&S management system; demonstrate diligence; gain assurance; etc. The benefits can be substantial. 189
- 191. What is NABL ? NABL specifies the general requirements for the competence to carry out tests and calibrations, including sampling. It covers testing and calibration performed using standard methods, non-standard methods, and laboratory-developed methods. 191
- 192. National Accreditation Board for Testing and Calibration Laboratories (NABL) is an autonomous body under the aegis of Department of Science & Technology, Government of India, and is registered under the Societies Act. NABL has been established with the objective to provide Government, Industry and Society in general with a scheme for third- party assessment of the quality and technical competence of testing and calibration laboratories. Government of India has authorized NABL as the sole accreditation body for Testing and Calibration laboratories. In order to achieve this objective, NABL provides laboratory accreditation services to laboratories that are performing tests / calibrations in accordance with NABL criteria based on internationally accepted standard for laboratory accreditation ISO/IEC 17025. These services are offered in a non-discriminatory manner and are accessible to all testing and calibration laboratories in India and abroad, regardless of their ownership, legal status, size and degree of independence. NABL has established its Accreditation System in accordance with ISO/IEC 17011:2004, which is followed internationally. NABL also compiles to the requirement of APLAC MR001 for the fulfillment of APLAC MRA and ILAC Arrangements. 192
- 193. Location of NABL Office: NABL Secretariat is functioning from its office situated at 3rd Floor, NISCAIR, 14, Satsang Vihar Marg, New Mehrauli Road – New Delhi 110067. NABL Secretariat administers and co-ordinates all activities of NABL including accreditation related activities for Testing and Calibration laboratories. Registered Office of NABL is located in Department of Science & Technology, Technology Bhavan, and New Mehrauli Road, New Delhi – 110016. Office Timing: The working days of NABL are from Monday to Friday. The office timings are from 9-00 a.m. to 5-30 p.m. 193
- 194. CONCEPT The concept of Laboratory Accreditation was developed to provide a means for third- party certification of the competence of laboratories to perform specific type(s) of testing and calibration. Laboratory Accreditation provides formal recognition of competent laboratories, thus providing a ready means for customers to find reliable testing and calibration services in order to meet their demands. Laboratory Accreditation enhances customer confidence in accepting testing / calibration reports issued by accredited laboratories. The globalization of Indian economy and the liberalization policies initiated by the Government in reducing trade barriers and providing greater thrust to exports makes it imperative for Accredited Laboratories to be at international level of competence. 194
- 195. Benefits of Accreditation: Potential increase in business due to enhanced customer confidence and satisfaction. Savings in terms of time and money due to reduction or elimination of the need for re-testing . Better control of laboratory operations and feedback to laboratories as to whether they have sound Quality Assurance System and are technically competent. 195
- 196. Increase of confidence in Testing / Calibration data and personnel performing work. Customers can search and identify the laboratories accredited by NABL for their specific requirements from the directory of Accredited Laboratories. Users of accredited laboratories will enjoy greater access for their products, in both domestic and international markets, when tested by accredited laboratories. 196
- 197. Types of Laboratory can seek Accreditation: Laboratories undertaking any sort of testing or calibration in the specified fields. Private or government laboratories. Small operations to large multi-field laboratories. Site facilities, temporary field operations and mobile laboratories. 197
- 198. TESTING LABORATORIES CALIBRATION LABORATORIES MEDICAL LABORATORIES Biological Chemical Electrical Electronics Fluid-Flow Mechanical Non-Destructive Testing Photometry Radiological Thermal Forensic Electro-Technical Mechanical Fluid Flow Thermal & Optical Radiological Clinical Biochemistry Clinical Pathology Haematology & Immunohaematology Microbiology & Serology Histopathology Cytopathology Genetics Nuclear Medicine (in- vitro tests only) PROFICIENCY TESTING PROVIDERS REFERENCE MATERIAL PRODUCERS Testing Calibration Medical Inspection Chemical Composition Biological & Clinical Properties Physical Properties Engineering Properties Miscellaneous Properties 198
- 199. 10 Step Approach To Accreditation Awareness Training Quality Policy & Objectives Finalization Gap Analysis Documentation / Process Design Documentation / Process Implementation Internal Audit Management Review Meeting Shadow Audit Corrective –Preventive Actions Final Certification Audit 199
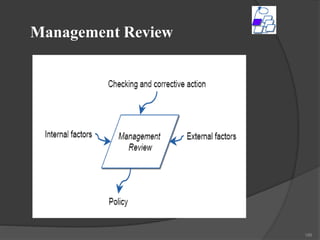
- 200. Step 1:- Awareness Training Separate training sessions for top management, middle management and junior level management. Creates a motivating environment throughout the organization for ISO 17025 implementation. 200
- 201. Step 2:-Quality Policy & Objectives Work shop with top management on development of quality policy. Work shop with top management and middle level functional management on development of quality objectives. 201
- 202. Step 3:-Gap Analysis Understanding of all the operations of the organization. Development of process map for the activities of the organization. Comparing existing operations with requirements of ISO 17025:2005 standard. 202
- 203. Step 4:-Documentation / Process Design Quality Manual Functional Procedures Work Instructions System Procedures Formats 203
- 204. Step 5:-Documentation / Process Implementation Work–shop on process / document implementation as per ISO 17025 requirements. Departmental / Individual assistance in implementing the new processes / documents. 204
- 205. Step 6:-Internal Audit Internal Audit Training & Examination (Optional). Successful employees / we carry out internal audit of the organization covering all the departments and operations. Suggest corrective and preventive actions for improvements in each of the audited departments. 205
- 206. Step 7:-Management Review Meeting Quality Policy & Objectives Results of internal audit Results of supplier evaluation Results of customer complaints Results of customer feedback etc. 206
- 207. Step 8:-Shadow Audit A replica of final certification audit. Finds degree of compliance with ISO 17025 standard. Gives an idea to the employees about the conduct of the final certification audit. 207
- 208. Step 9:-Corrective – Preventive Actions On the basis of shadow audit conducted in the last step, all the non-conformities will be assigned corrective and preventive actions. A check will ensure that all the NCs are closed and the organization is ready for the final certification audit. 208
- 209. Step 10:-Final Certification Audit Upon completion of various stages of accreditation audit, the audit, your organization will be awarded accreditation. 209
- 211. 5. NABL Certification Differ from ISO 9000 Certification: ISO 9000 Certification is on Quality System Management only whereas the NABL Accreditation provides formal recognition of technical competence of the laboratories, thus providing a ready means for customers to find reliable testing and calibration services in order to meet their demands as well as the Quality system. Accreditation is a higher level activity than system certification. Laboratories can be checked and certified for their compliance to international management system standards such as ISO 9000. This involves the auditing of an organization’s quality management system. Although this will give you confidence of the laboratory’s quality system, it tells you nothing about its technical competence or its ability to provide reliable and accurate test data that will be accepted by your customers and trading partners. 211
- 212. Proper technical evaluation requires the use of technical experts who can assess the laboratory against internationally accepted criteria. These criteria are embraced globally in a document called ISO/IEC 17025. Accreditation bodies may also apply additional technical requirements for evaluating a laboratory, as per requirements of different technical fields 6. Proficiency Testing Programmes Organized by NABL: All NABL accredited testing and calibration laboratories are required to participate in Proficiency Testing Programmes conducted by NABL or the nodal organizations appointed by NABL. Accredited and applicant laboratories are required to approach NABL Co-coordinator for Proficiency Testing programs or the nodal laboratories appointed by NABL whenever a PT programme for a specific testing / calibration is organized by NABL. For calibration laboratories, NPL, Delhi is the main nodal laboratory.212
- 213. Laboratories are also expected to participate (as far as available and practicable) in international Inter- Laboratory Comparison / Proficiency Programmes conducted by APLAC, EA or equivalent organizations. NABL keeps the accredited laboratories informed about all such international programmes through NABL newsletter / APLAC newsletter. It is essential for all its accredited laboratories to participate in International / Regional Proficiency Testing Programmes including APLAC in a manner so that all major areas of scope of accreditation are covered in a cycle of 4 years. This of course applies to those special areas where Inter-Laboratory Proficiency Testing Programmes are not available. All applicant laboratories are required to successfully participate in at least one Inter - Laboratory Proficiency Testing in accordance with ISO/IEC Guide-43. For this purpose all alternative techniques covered in ISO/IEC Guide-43 will be acceptable. 213
- 214. 7.Criteria for using NABL Symbol: All NABL accredited laboratories are expected to use NABL symbol on their letterhead, test / calibration reports and any other relevant documents. NABL symbol shall be used for the purpose of identifying correctly and unambiguously the test / calibration services accredited by NABL. While using the symbol it shall be ensured by the laboratory that design and its manifestations are not distorted, it can be reproduced in any single color (preferably black) and any size. It shall be responsibility of the accredited laboratory that the use of symbol does not misrepresent the scope of accredited testing / calibration services. In case where the accreditation sought and granted do not cover all the activities of the laboratory's services care should be exercised to restrict the use of symbol only to those accredited activities. The letterheads and publicity materials, brochures, test / calibration reports of the accredited laboratory bearing the NABL symbol shall cover only the test results under accredited category. 214
- 215. Accredited laboratories shall not authorize the use of symbol for tests / calibration services sub-contracted to other laboratories, which are not accredited by NABL. In case of complaints in this regard from users and other laboratories, NABL shall get the same examined by a committee and take appropriate action. 215
- 216. 8. International Recognition of NABL: NABL maintains its linkages with the international bodies like International Laboratory Accreditation Co-operation (ILAC) and Asia Pacific Laboratory Accreditation Co-operation (APLAC). NABL is a full member of both ILAC and APLAC. NABL is a signatory to ILAC as well as APLAC Mutual Recognition Arrangements (MRA), which is based on mutual evaluation and acceptance of other MRA Partner laboratory accreditation systems. Such international arrangements facilitate acceptance of test / calibration results between countries to which MRA partners represent. In order to achieve the objective of the acceptance of test / calibration data across the national borders, NABL operates and is committed to update its laboratory accreditation system as per international norms. A current NABL criterion for Laboratory Accreditation is ISO/IEC 17025 standard for testing and calibration laboratories and ISO 15189:2003 for medical laboratories. 216
- 217. 8.1 Asia Pacific Laboratory Accreditation Cooperation (APLAC): It is the group of accreditation bodies in the Asia Pacific region responsible for accrediting calibration, testing and inspection facilities. This reduces the need for re-testing of products and therefore saves time and money. APLAC has active programmed for the development of technical guidance documents, inter-laboratory comparisons (proficiency testing), and for training of laboratory assessors. APLAC is recognized by Asia Pacific Economic Cooperation (APEC) member economies as a Specialist Regional Body (SRB). 217
- 218. 8.2 ISO/IEC 17025: ISO/IEC 17025 is the basis for competency of testing and calibration laboratories. Accreditation to ISO/IEC 17025 requires that: The laboratory has a quality system meeting requirements of ISO 9001. The lab facility has adequate equipment to perform its testing or calibration tasks. The lab facility has adequate laboratory personnel with the competence to perform the calibration and testing. In addition most accreditation schemes in the U.S. require proficiency testing amongst the laboratories. Therefore, ISO/IEC 17025 is recognition of laboratory competence, while ISO 9001 alone is simply recognition of conformance to a quality system. 218
- 219. 8.3 International Laboratory Accreditation Cooperation (ILAC): ILAC first started as a conference in 1977 with the aim of developing international cooperation for facilitating trade by promotion of the acceptance of accredited test and calibration results. In 1996, ILAC became a formal cooperation with a character to establish a network of mutual recognition agreements among accreditation bodies that would fulfill this aim. 219
- 220. 220
- 221. Electronic Records; Electronic Signatures (21 CFR Part 11). 221
- 222. 21 CFR Part 11 Part 11 applies to records in electronic form that are created, modified, maintained, archived, retrieved, or transmitted under any records requirements set forth in Agency regulations. Part 11 also applies to electronic records submitted to the Agency under the Federal Food, Drug, and Cosmetic Act (the Act) and the Public Health Service Act (the PHS Act), even if such records are not specifically identified in Agency regulations. 222
- 223. FDA's guidance documents, including this guidance, do not establish legally enforceable responsibilities. In March of 1997, FDA issued final part 11 regulations that provide criteria for acceptance by FDA, under certain circumstances, of electronic records, electronic signatures, and handwritten signatures executed to electronic records as equivalent to paper records and handwritten signatures executed on paper. After part 11 became effective in August 1997, significant discussions ensued among industry, contractors, and the Agency concerning the interpretation and implementation of the regulations. 223
- 224. 21 CFR Part 11; Electronic Records; Electronic Signatures, Validation 21 CFR Part 11; Electronic Records; Electronic Signatures, Glossary of Terms 21 CFR Part 11; Electronic Records; Electronic Signatures, Time Stamps 21 CFR Part 11; Electronic Records; Electronic Signatures, Maintenance of Electronic Records 21 CFR Part 11; Electronic Records; Electronic Signatures, Electronic Copies of Electronic Records 224
- 225. Controls and Requirements limiting system access to authorized individuals use of operational system checks use of authority checks use of device checks determination that persons who develop, maintain, or use electronic systems have the education, training, and experience to perform their assigned tasks establishment of and adherence to written policies that hold individuals accountable for actions initiated under their electronic signatures appropriate controls over systems documentation controls for open systems corresponding to controls for closed systems bulleted above requirements related to electronic signatures 225
- 226. Approaches of Part 11 Narrow Interpretation of Scope Under the narrow interpretation of the scope of part 11, with respect to records required to be maintained under predicate rules or submitted to FDA, when persons choose to use records in electronic format in place of paper format, part 11 would apply. when persons use computers to generate paper printouts of electronic records, and those paper records meet all the requirements of the applicable predicate rules and persons rely on the paper records to perform their regulated activities, FDA would generally not consider persons to be "using electronic records in lieu of paper records" 226
- 227. Definition of Part 11 Records Records that are required to be maintained under predicate rule requirements and that are maintained in electronic format in place of paper format Electronic signatures that are intended to be the equivalent of handwritten signatures, initials, and other general signings required by predicate rules. Part 11 signatures include electronic signatures that are used, for example, to document the fact that certain events or actions occurred in accordance with the predicate rule (e.g. approved, reviewed, and verified). 227
- 228. Validation Decision to validate computerized systems, and the extent of the validation, take into account the impact the systems have on your ability to meet predicate rule requirements. You should also consider the impact those systems might have on the accuracy, reliability, integrity, availability, and authenticity of required records and signatures. Even if there is no predicate rule requirement to validate a system, in some instances it may still be important to validate the system. 228
- 229. Audit Trail The Agency intends to exercise enforcement discretion regarding specific part 11 requirements related to computer-generated, time-stamped audit trails. Persons must still comply with all applicable predicate rule requirements related to documentation of, for example, date, time, or sequencing of events, as well as any requirements for ensuring that changes to records do not obscure previous entries. Audit trails can be particularly appropriate when users are expected to create, modify, or delete regulated records during normal operation. 229
- 230. Legacy Systems This means that the Agency does not intend to take enforcement action to enforce compliance with any part 11 requirements if all the following criteria are met for a specific system: The system was operational before the effective date. The system met all applicable predicate rule requirements before the effective date. The system currently meets all applicable predicate rule requirements. You have documented evidence and justification that the system is fit for its intended use (including having an acceptable level of record security and integrity, if applicable). 230
- 231. Copies of Records I. Producing copies of records held in common portable formats when records are maintained in these formats II. Using established automated conversion or export methods, where available, to make copies in a more common format (examples of such formats include, but are not limited to PDF, XML, or SGML) the copying process used produces copies that preserve the content and meaning of the record. If you have the ability to search, sort, or trend part 11 records, copies given to the Agency should provide the same capability if it is reasonable and technically feasible. You should allow inspection, review, and copying of records in a human readable form at your site using your hardware and following your established procedures and techniques for accessing records. 231
- 232. Record Retention The Agency intends to exercise enforcement discretion with regard to the part 11 requirements for the protection of records to enable their accurate and ready retrieval throughout the records retention period. Persons must still comply with all applicable predicate rule requirements for record retention and availability In addition, paper and electronic record and signature components can co-exist (i.e., a hybrid situation) as long as predicate rule requirements are met and the content and meaning of those records are preserved232
- 233. Reference http://www.nabl-india.org. http://www.fda.org. http://www.ilac.org/arrangement.htm. http://www.aplac.org/members/signatories_mra.htm. Food and Drug Administration References 1. Glossary of Computerized System and Software Development Terminology (Division of Field Investigations, Office of Regional Operations, Office of Regulatory Affairs, FDA 1995) (http://www. fda.gov/ora/inspect_ref/igs/gloss.html) 2. General Principles of Software Validation; Final Guidance for Industry and FDA Staff (FDA, Center for Devices and Radiological Health, Center for Biologics Evaluation and Research, 2002) (http://www.fda.gov/cdrh/comp/guidance/938.html) 3. Guidance for Industry, FDA Reviewers, and Compliance on Off-The-Shelf Software Use in Medical Devices (FDA, Center for Devices and Radiological Health, 1999) (http://www.fda.gov/cdrh/ode/guidance/585.html) 4. Pharmaceutical CGMPs for the 21st Century: A Risk-Based Approach; A Science and Risk-Based Approach to Product Quality Regulation Incorporating an Integrated Quality Systems Approach (FDA 2002) (http://www.fda.gov/oc/guidance/gmp.html) 233
- 234. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) Center for Devices and Radiological Health (CDRH) Center for Food Safety and Applied Nutrition (CFSAN) Center for Veterinary Medicine (CVM) Office of Regulatory Affairs (ORA) August 2003 Pharmaceutical CGMPs 234
Editor's Notes
- #25: For an example, we can get the pen. Pen point, case,
- #29: Related to define : flow charts(shows the visual representation of the processes), cause/effect diagrams (shows the possible causes of a problem), check sheets, pareto analysis (shows the frequency of the problem).
- #48: Application of these clauses means
- #49: ACSK village meetings No specific clauses
- #50: No isolated process All process be synch with each other