Phase Diagram & Heat Treatment Of Metals
- 1. Dr. Pulak M. Pandey http:/ / paniit.iitd.ac.in/ ~ pmpandey
- 2. Metals and alloys may not posses all the desired properties in the finished product. Alloying and heat treatment are two methods which are extensively used for controlling material properties. In heat treatment, the microstructures of materials are modified. The resulting phase transformation influences mechanical properties like strength, ductility, toughness, hardness and wear resistance. Purpose of heat treatment is to increase service life of a product by increasing its strength of hardness, or prepare the material for improved manufacturability The basis of change in properties is phase or equilibrium diagrams
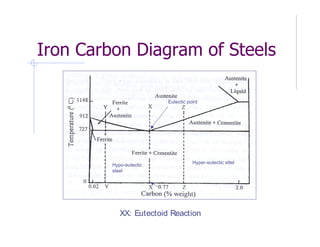
- 4. FE-C Phase Diagram In the phase diagram Carbon percentage is shown up to 6% only since commercially pure iron contains up to 0.008% C, Steels up to 2.11% C and C.I.s up to 6.67% C. Pure iron melts at 1583o C. When it cools first it forms delta ferrite, then austenite and finally alpha ferrite. Alpha ferrite or ferrite is a solid solution of BCC iron with a maximum solid solubility of 0.022% C at a temperature of 727oC. Delta ferrite has no practical significance as it is stable only at high temperatures. Ferrite (derived from Latin word Ferrum) is relatively soft and ductile and is magnetic up to 768oC. Iron, between 1394 to 912oC, undergoes transformation form BCC to FCC structure to give Gamma iron, commonly known as Austenite. The solid solubility of Austenite is much higher than ferrite and is up to 2.11 % C. Austenite is denser than ferrite and more ductile at higher temperatures. Steel in austenitic form is non-magnetic. Cementite, represented by right hand boundary of the phase diagram, is 100% iron carbide with 6.67% C. Cementite is very hard and brittle inter-metallic compound.
- 5. Eutectic point Hyper-eutectic sttel Hypo-eutectic steel XX: Eutectoid Reaction
- 6. Eutectoid Reaction When iron containing 0.77%C, is cooled from 1100oC, in the austenitic phase (line XX), a reaction takes place, when the temperature reaches 727oC, which converts it to ferrite (BCC) and cementite. This reaction is called Eutectoid Reaction. The resultant microstructure of eutectoid steel is called Pearlite which contains alternate layers of ferrite and cementite. Mech. Prop. Of Pearlite is therefore in between soft and ductile ferrite and hard and brittle cementite.
- 7. Hypo-eutectoid Steel Similarly when the carbon content is less than 0.77% (line YY) the material is entirely austenitic at higher temperature, but cooling it enters the region of stable ferrite and austenite. At 727oC the austenite is of eutectoid composition and has 0.77%C, and on further cooing the remaining austenite transforms into pearlite. The resulting structure is proeutectoid ferrite and pearlite.
- 8. Hyper-eutectoid Steel When steel cool along line ZZ, the proeutectoid phase is ferrite than austenite. As the carbon-rich phase forms, the remaining austenite decreases in carbon content and reaches eutectoid composition at 727oC. Any remaining austenite, transforms into pearlite below 727oC. The resulting structure has continuous network of cementite which causes the material to be extremely brittle. Point to be noted here that these transformations are obtained during slow cooling, however by rapid cooling entirely different results are obtained since sufficient time is not available for phase reaction to occur.
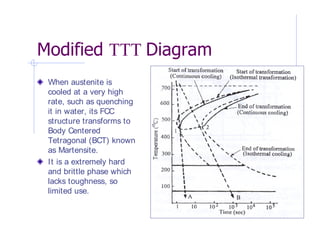
- 9. Time-Temperature-Transformation (TTT) Diagram for Steel Pearlite is produced if cooling rate is slow like in air or in a furnace. Fine pearlite is harder and less ductile than coarse pearlite. Bainite is a very fine microstructure, consisiting of ferrite and cementite, somewhat like Pearlite but have different morphology.This phase is stronger and more ductile than pearlite. TTT diagram of Eutectic Steel
- 10. When austenite is cooled at a very high rate, such as quenching it in water, its FCC structure transforms to Body Centered Tetragonal (BCT) known as Martensite. It is a extremely hard and brittle phase which lacks toughness, so limited use.
- 11. Microstructures Unit cells of FCC, BCC, BCT structures Pearlite Martensite 99% Matensite
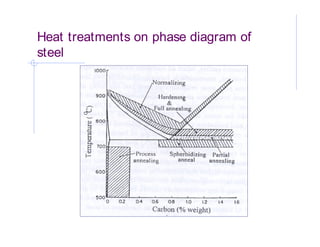
- 12. Hardening Hardening is performed to impart strength and hardness to alloys by heating up to a certain temperature, depending on the material, and cooling it rapidly. Steel is heated up to austenitic region and held there until its carbon is dissolved, and then cooled rapidly, the carbon does not get sufficient time to escape and get dissipated in the lattice structure. This helps in locking the dislocation movements when stresses are applied. Quenching is performed to cool hot metal rapidly by immersing it in brine (salt water), water, oil, molten salt, air or gas. Quenching sets up residual stresses in the workpiece and sometimes results in cracks. Residual stresses are removed by another process called annealing.
- 13. Annealing Annealing is performed to reduce hardness, remove residual stresses, improve toughness, restore ductility, and to alter various mechanical, electrical or magnetic properties of material trough refinement of grains. Cooling rate is very slow around 10oC per hour. Process is carried out in a controlled atmosphere of inert gas to avoid oxidation. Partial annealing is incomplete annealing and there is partial phase transformation however in sub-critical annealing there is no phase transformation. Used to achieve ductility in work hardened steels.
- 14. Normalizing The process is similar to annealing and is carried out to avoid excessive softness in the material. The material is heated above austenitic phase and then cooled in air . This gives relatively faster cooing and hence enhanced hardness and less ductility. In this process, austenite is decomposed in ferrite and carbide at relatively lower temperature and fine pearlite is produced. Normalizing is less expensive than annealing. In normalization variation in properties of different sections of a part is achieved. The selection of heat treatment operations is strongly influenced by the carbon content in th esteel.
- 15. Heat treatments on phase diagram of steel
- 16. Tempering Martensite is very hard and brittle. Tempering is applied to hardened steel to reduce brittleness, increase ductility, and toughness and relieve stresses in martensite structure. In this process, the steel is heated to lower critical temperature keeping it there for about one hour and then cooled slowly at prescribed rate. This process increses ductility and toughness but also reduces hardness, strength and wear resistance marginally. Increase in tempering temperature lowers the hardness.
- 17. Surface Hardening Heat treatment methods in general change the properties of entire material. Hardening improves wear resistance of material but lowers impact resistance and fatigue life. Therefore sometimes there is requirement of surface hardening Two methods are used, first is heating and cooing to get required phase, and second is thermo-chemical treatment. Induction heating Flame hardening High frequency resistance heating Laser beam hardening Electron beam hardening Carburizing Nitriding Cyanding
- 18. This document was created with Win2PDF available at http://www.daneprairie.com. The unregistered version of Win2PDF is for evaluation or non-commercial use only.