Power point akl
- 1. WELCOME SUBMITTED BY AKHIL CHANDRAN PHYSICAL SCIENCE
- 3. What are the 3 major parts of an atom? • Proton • Neutron • Electron
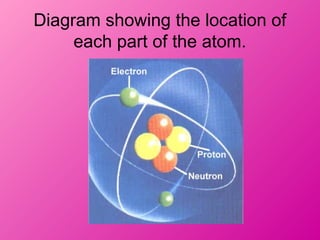
- 4. Diagram showing the location of each part of the atom.
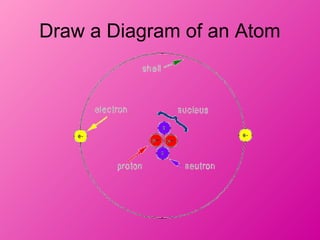
- 5. Draw a Diagram of an Atom
- 6. PROTON • Protons are positively charged particles found in the atomic nucleus. Protons were discovered by Ernest Rutherford.. • Experiments done in the late 1960's and early 1970's showed that protons are made from other particles called quarks. Protons are made from two 'up' quarks and one 'down' quark.
- 7. NEUTRON • Neutrons are uncharged particles found in the atomic nucleus. Neutrons were discovered by James Chadwick in 1932. • Experiments done in the late 1960's and early 1970's showed that neutrons are made from other particles called quarks. Neutrons are made from one 'up' quark and two 'down' quarks.
- 8. ELECTRON Electrons are negatively charged particles that surround the atom's nucleus. Electrons were discovered by J. J. Thomson in 1897. Electrons determine properties of the atom. Chemical reactions involve sharing or exchanging electrons.
- 9. NUCLEUS The nucleus is the central part of an atom. It is composed of protons and neutrons. The nucleus contains most of an atom's mass. It was discovered by Ernest Rutherford in 1911.
- 10. THANK YOU