Ppt chemical reactions
- 2. Chemical Reaction What is a chemical reaction? • Atoms rearrange to create one or more new compounds. • In other words, old bonds break and new bonds form.
- 4. Chemical Reaction OLD NEW Reactants Products
- 5. Chemical Equation Reactants Products YEILD OR = Reactants and products can be represented by using chemical formulas or compound names.
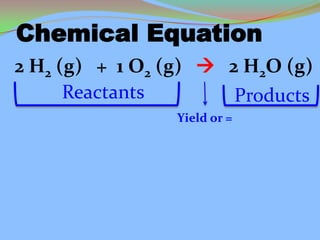
- 6. Chemical Equation 2 H2 (g) + 1 O2 (g) 2 H2O (g) Reactants Products Yield or =
- 7. Chemical Reactions can 1.Absorb or release energy. 1.Fast or Slow
- 8. Absorb energy or release energy?
- 9. Exothermic- release of energy. Exo- Think exit! Thermic – heat Endothermic- absorption of energy Endo- Taken in Thermic – heat Chemical Reactions can be…
- 11. 2. Fast or slow The speed of a chemical reaction is different for all! Depends on the reactants. Chemical Reactions can be…
- 12. Reaction Rate Activity- the speed a reaction going from reactants to products.
- 13. Law of Conservation of Matter The law of conservation of matter states that matter (mass) can neither be created nor destroyed. It can, however, can be rearranged. In a chemical reaction, the mass of the reactants must equal the mass of the products.
- 14. Law of Conservation of Matter The law of conservation of matter states that matter (mass) can neither be created nor destroyed. It can, however, can be rearranged. In a chemical reaction, the mass of the reactants must equal the mass of the products. WHAT ??????
- 15. Think of a Balance Everything must be equal. When matter goes through a physical or chemical change, the amount (or mass) of the substances that you begin with must equal the amount (or mass) of the substances that you end with. BEFORE MASS MUST = THE AFTER MASS! WHY you ask!!!!!!!
- 16. How do we balance chemical equations?!?!?! 1.USE A PENCIL!! 2.Identify reactants and products 3. Make a Table!!!
- 17. EXAMPLE: H2O2 O2 + H2O How do we balance chemical equations?!?!?! How many of each element is in the reactants? How many of each element is in the products? Element w/o coeff. X coeff. Total Element w/o coeff. X coeff. Total
- 18. EXAMPLE: KClO3KCl + O2 How do we balance chemical equations?!?!?! How many of each element is in the reactants? How many of each element is in the products? Element w/o coeff. X coeff. Total Element w/o coeff. X coeff. Total
- 19. EXAMPLE: CH4 + O2 CO2 + H2O How do we balance chemical equations?!?!?! How many of each element is in the reactants? How many of each element is in the products? Element w/o coeff. X coeff. Total Element w/o coeff. X coeff. Total
- 21. 1. Synthesis- Two or more simple substances are combined to form one new and more complex substance. Types of Reactions A + B AB
- 22. 1. Synthesis- Two or more simple substances are combined to form one new and more complex substance. Types of Reactions A + B AB 2Na + Cl2 2NaCl
- 23. 2. Decomposition Reaction- A more complex substance breaks down into its more simple parts. Types of Reactions AB A + B
- 24. 2. Decomposition Reaction- A more complex substance breaks down into its more simple parts. Types of Reactions AB A + B H2O2 O2 + H2O
- 25. 3. Single-Replacement- A free uncombined element replaces another combined element in a compound. Types of Reactions AB + C AC + B
- 26. 3. Single-Replacement- A free uncombined element replaces another combined element in a compound. Types of Reactions AB + C AC + BLiCl + Br2LiBr + Cl2
- 27. 4. Double Replacement Reaction- The reacting parts of two compounds switch places to form two new compounds. Types of Reactions AB + CD AD + BC
- 28. 4. Double Replacement Reaction- The reacting parts of two compounds switch places to form two new compounds. Types of Reactions AB + CD AD + BC CaF2 + LiSO4 CaSO4 + LiF
- 29. 5. Combustion Reaction- OR Burning Reaction Involves the combination of a substance with oxygen to give off heat and light. Types of Reactions AB + O2 Heat+ Light
- 30. 5. Combustion Reaction- OR Burning Reaction Involves the combination of a substance with oxygen to give off heat and light. Types of Reactions AB + O2 Heat+ Light CH4 + O2 CO2 + H2O
- 31. Types of Reactions Use the code below to classify each reaction. S = Synthesis D = Decomposition SR = Single Replacement DR = Double Replacement ____ P + O2 → P4O10 ____ Mg + O2 → MgO ____ HgO → Hg + O2____ Al2O3 → Al + O2 ____ Cl2 + NaBr → NaCl + Br2 ____ H2 + N2 → NH3