Properties of Gases
- 1. Chemistry Unit 10: The Gas Laws
- 2. The Atmosphere is all around us an “ocean” of gases mixed together Composition nitrogen (N2)………….. ~78% oxygen (O2)…………… ~21% argon (Ar)……………... ~1% carbon dioxide (CO2)… ~0.04% Trace amounts of: He, Ne, Rn, SO2, water vapor (H2O)……. ~0.1% CH4, NxOx, etc.
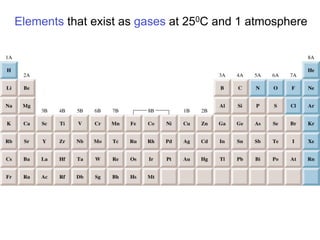
- 3. Elements that exist as gases at 250C and 1 atmosphere
- 5. Gases have mass. • Gases seem to be weightless, but they are classified as matter, which means they have mass. • The density of a gas – the mass per unit of volume – is much less than the density of a liquid or solid, however.
- 6. Gases have mass. It’s this very low density that allows us to be able to walk through the room without concerning ourselves with air resistance. Since it is so easy to “swim” across the room we don’t put much thought into the mass of a gas. Really it is only noticeable if we have a large collection of gas in a container.
- 7. Physical Characteristics of Gases • Gases assume the volume and shape of their containers. • Gases are the most compressible state of matter. • Gases will mix evenly and completely when confined to the same container. • Gases have much lower densities than liquids and solids.
- 8. Compressibility Gases can expand to fill its container, unlike solids or liquids The reverse is also true: They are easily compressed, or squeezed into a smaller volume Compressibility is a measure of how much the volume of matter decreases under pressure
- 9. Variables that describe a Gas There are FOUR variables used to describe gases: 1. pressure (P) 2. volume (V) 3. temperature (T) 4. amount (n)
- 10. 1. Pressure of Gas Pressure results from the collisions between gas molecules and the walls of the container they are in. More molecules means more collisions which means more pressure. Gases naturally move from areas of high pressure to low pressure, because there is empty space to move into – a spray can is example.
- 11. Units of Pressure The standard unit for pressure is ATMOSPHERE or atm Other units include: kPa = kilopascal mmHg = millimeter of Mercury torr = Torrricelli psi = pounds per square inch
- 12. Convert between Pressure units 1 atm equals... 101.325 kPa 760 mm Hg 760 torr 14.69 psi
- 13. 2. Volume of Gas In a smaller container, the molecules have less room to move. The particles hit the sides of the container more often. As volume decreases, pressure increases. (think of a syringe) Thus, volume and pressure are inversely related to each other
- 14. Units of Volume The standard unit for volume is the... LITER or L *There are 1,000 mL in 1 L
- 15. 3. Temperature of Gas Temperature is a measurement of the amount of Kinetic Energy the gas molecules contain Raising the temperature of a gas increases the pressure, if the volume is held constant. (Temp. and Pres. are directly related) The molecules hit the walls harder, and more frequently! Should you throw an aerosol can into a fire? What could happen?
- 16. Units of Temperature The standard unit for temperature is... Kelvin or K Other units include: Degrees Celsius = oC Degrees Fahrenheit = oF
- 17. Absolute Zero The theoretical temperature at which all kinetic motion completely stops. Equal to 0 K or -273 oC Conversions K = °C + 273 °C = K – 273
- 18. 4. Amount of Gas When we inflate a balloon, we are adding gas molecules. Increasing the number of gas particles increases the number of collisions thus, the pressure increases The standard unit for the amount of gas molecules is the: MOLE (mol)
- 19. And now, we pause for this commercial message from STP OK, so it’s really not THIS kind of STP… STP in chemistry stands for Standard Temperature and Pressure Standard Pressure = STP allows us to compare 1 atm amounts of gases between different pressures and Standard Temperature temperatures = 0 oC or 273 K
- 20. Kinetic Molecular Theory The theory states that the tiny particles in all forms of matter in all forms of matter are in constant motion. This theory is used to explain the behaviors common among gases There are 3 basic assumptions of the KMT as it applies to gases.
- 21. Kinetic Molecular Theory of Gases Three basic assumptions of the kinetic theory as it applies to gases: #1. A gas is composed of small, particles that have mass- usually molecules or atoms. They have... Insignificant volume; relatively far apart from each other No attraction or repulsion between particles
- 22. Kinetic Molecular Theory of Gases #2. Particles in a gas move rapidly in constant random motion Move in straight paths, changing direction only when colliding with one another or other objects Average speed of O2 in air at 20 oC is an amazing 1700 km/h!
- 23. Kinetic Molecular Theory of Gases #3. Collisions are perfectly elastic- meaning kinetic energy is transferred without loss from one particle to another- the total kinetic energy remains constant
- 24. Summary of The Kinetic Molecular Theory -- explains why gases behave as they do 1. …are so small that they are assumed to have zero volume 2. …are in constant, straight-line motion 3. …experience elastic collisions in which no energy is lost 4. …have no attractive or repulsive forces toward each other 5.…have an average kinetic energy (KE) that is proportional to the absolute temp. of gas (i.e., Kelvin temp.) as Temp. , KE
- 25. Kinetic Molecular Theory of Gases Gas Pressure – defined as the force exerted by a gas per unit surface area of an object Due to: a) force of collisions, and b) number of collisions No particles present? Then there cannot be any collisions, and thus no pressure – called a vacuum
- 26. Kinetic Molecular Theory of Gases Atmospheric pressure results from the collisions of air molecules with objects Decreases as you climb a mountain because the air layer thins out, meaning less particles, as elevation increases Barometer is the measuring device for atmospheric pressure, which is dependent upon weather & altitude
- 27. Atmospheric pressure changes with altitude: As altitude , pressure . barometer: device to measure air pressure vacuum mercury air (Hg) pressure
- 28. Measuring Pressure The first device for measuring atmospheric pressure was developed by Evangelista Torricelli during the 17th century. The device was called a “barometer” Baro = weight Meter = measure Torricelli
- 29. An Early Barometer The normal pressure due to the atmosphere at sea level can support a column of mercury that is 760 mm high.
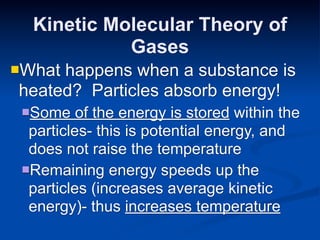
- 30. Kinetic Molecular Theory of Gases Whathappens when a substance is heated? Particles absorb energy! Some of the energy is stored within the particles- this is potential energy, and does not raise the temperature Remaining energy speeds up the particles (increases average kinetic energy)- thus increases temperature
- 31. Kinetic Molecular Theory of Gases Anincrease in the average kinetic energy of particles causes the temperature to rise. As it cools, the particles tend to move more slowly, and the average K.E. declines. Is there a point where they slow down enough to stop moving?
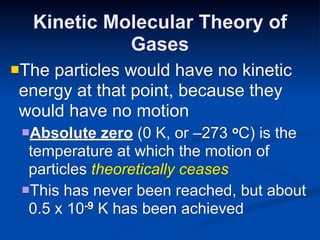
- 32. Kinetic Molecular Theory of Gases Theparticles would have no kinetic energy at that point, because they would have no motion Absolute zero (0 K, or –273 oC) is the temperature at which the motion of particles theoretically ceases This has never been reached, but about 0.5 x 10 -9 K has been achieved
- 33. •Diffusion: describes the mixing of gases. The rate of diffusion is the rate of gas mixing. •Molecules move from areas of high concentration to low concentration.
- 34. Effusion: a gas escapes through a tiny hole in its container -Think of a nail in your car tire…