Ps5 atoms and molecules
- 1. Atoms and molecules PS5 2.08
- 2. All matter is made from tiny particles called atoms. Atoms are so tiny that a million of them together would still be too small to see without a microscope.
- 3. All matter is made from tiny particles called atoms. Atoms are so tiny that a million of them together would still be too small to see without a microscope.
- 4. Atoms all have a center called the nucleus. It’s like a sun in the middle of a solar system. nucleus
- 5. The nucleus of every atom contains tiny subatomic particles called protons and in some atoms there are also neutrons . protons neutrons
- 6. Orbiting around the nucleus, like very fast planets, are electrons . electrons There are about the same number of protons and electrons in each atom.
- 8. What are the three types of subatomic particles that atoms are made from?
- 9. What are the three types of subatomic particles that atoms are made from? protons
- 10. What are the three types of subatomic particles that atoms are made from? protons neutrons
- 11. What are the three types of subatomic particles that atoms are made from? protons neutrons electrons
- 12. In the nucleus (center). Where in the atom are protons found?
- 13. In the nucleus (center). Where in the atom are protons found?
- 14. In the nucleus (center). Where in the atom are protons found? Neutrons are found in the nucleus, too.
- 15. Where in the atom are electrons found?
- 16. Where in the atom are electrons found? Electrons orbit very quickly outside the nucleus .
- 18. There are 90 natural elements. Elements are what we call the different kinds of atoms. The thing that makes elements different from each other is the number of protons in the nucleus.
- 19. Hydrogen has one proton
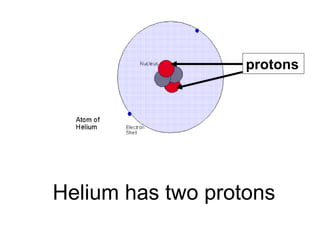
- 20. Helium has two protons protons
- 21. Carbon has 6 protons
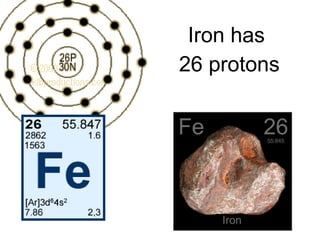
- 22. Iron has 26 protons
- 23. Gold has 79 protons
- 24. The Periodic Table of Elements lists all the elements. They are in order according to their number of protons.
- 25. What is the chart that scientists use to list all the elements?
- 26. What is the chart that scientists use to list all the elements? The Periodic Table of Elements
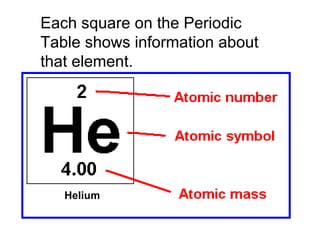
- 27. Each square on the Periodic Table shows one element.
- 28. Each square on the Periodic Table shows information about that element. Helium
- 29. The different number of protons in elements (the atomic number) gives each one different properties or characteristics.
- 30. Hydrogen, with one proton, is a very light gas.
- 31. There were once huge airships filled with hydrogen that could float like balloons.
- 32. But hydrogen gas has the property of being highly flammable. It can explode with one little spark.
- 33. Helium, with two protons, is also a very light gas that works well in balloons. But helium is safe. It won’t burn at all.
- 34. What could happen if party balloons were filled with hydrogen gas instead of helium?
- 35. What could happen if party balloons were filled with hydrogen gas instead of helium?
- 36. They could have made new airships filled with helium instead of hydrogen.
- 37. But after the Hindenburg exploded people didn’t want to fly in gas filled airships anymore.
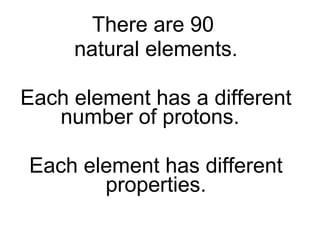
- 38. There are 90 natural elements. Each element has a different number of protons. Each element has different properties.
- 39. What do we call the different types of atoms?
- 40. What do we call the different types of atoms? Elements
- 41. What do we call the different types of atoms? Elements There are 90 different natural elements.
- 42. What two things make the elements different from each other?
- 43. What two things make the elements different from each other? the number of protons
- 44. What two things make the elements different from each other? number of protons properties (how it acts)
- 45. There are 90 different elements but there are a lot more than 90 different kinds of “stuff.”
- 46. There are 90 different elements but there are a lot more than 90 different kinds of “stuff.” There are millions of different kinds of stuff.
- 47. There are 90 different elements but there are a lot more than 90 different kinds of “stuff.” There are millions of different kinds of stuff. How can they all come from only 90 elements?
- 48. Just like 26 different letters in the alphabet can be put together in different ways to make thousands of words….. ABCDEFGHIJKLMNOPQRSTUVWXYZ c + a + t = cat
- 49. 90 different elements can be put together in different ways to make millions of different kinds of stuff….. Hydrogen + Oxygen = water (H 2 O)
- 50. The new stuff made when two or more atoms are joined is called a compound . The smallest particle of a compound is called a molecule.
- 51. Two atoms of hydrogen
- 52. Together with one atom of oxygen
- 53. Makes one molecule of a compound called water (H 2 O).
- 54. Water molecules can be drawn different ways, but the all have two hydrogen atoms and one oxygen atom of water (H 2 O)
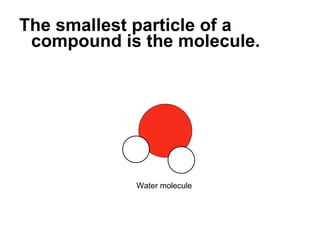
- 55. The smallest particle of a compound is the molecule. If you break a molecule apart, it’s not water anymore. Water molecule
- 56. The smallest particle of a compound is the molecule. If you break a water molecule apart , it’s not water anymore. Water molecule
- 57. The smallest particle of a compound is the molecule. If you break a water molecule apart, it’s not water anymore. Oxygen atom Hydrogen atoms
- 58. What is the smallest particle of an element?
- 59. What is the smallest particle of an element? An atom Oxygen atom Hydrogen atom
- 60. What is the smallest particle of a compound?
- 61. What is the smallest particle of a compound? A molecule Water molecule
- 62. How many atoms are in a molecule?
- 63. How many atoms are in a molecule? 2 or more
- 65. A compound can have totally different properties than the elements that make it.
- 66. One property of hydrogen is that it is highly flammable (burns easily).
- 67. Oxygen is an element that also has the property of flammability.
- 68. But the compound made from hydrogen and oxygen is the least flammable thing there is. Hydrogen + oxygen H 2 O Water
- 69. But a compound made from hydrogen and oxygen is the least flammable thing there is. We use water to put fires out. Hydrogen + oxygen = water
- 70. But a compound made from hydrogen and oxygen is the least flammable thing there is. We use water to put fires out. Remember: A compound can have different properties than the elements that made it.
- 71. Sodium is an element. Sodium is also a metal.
- 72. A property of pure sodium is that it reacts violently when it comes in contact with water.
- 73. Chlorine is an element. Pure chlorine is a poisonous green gas. You sure wouldn’t want pure chlorine in your food.
- 74. Chlorine is an element. Pure chlorine is a poisonous green gas. You sure wouldn’t want pure chlorine in your food.
- 75. Chlorine is an element. Pure chlorine is a poisonous green gas. If something has the property of being poisonous, you sure wouldn’t want to put it in your food.
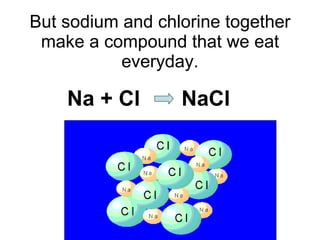
- 76. But sodium and chlorine together make a compound that we eat everyday. Na + Cl NaCl
- 77. Sodium chloride (NaCl) is the chemical we call salt.
- 78. Sodium chloride (NaCl) is the chemical we call salt. Remember: A compound may have properties that are very different from the elements that join together to make it.
- 79. Sometimes two or more compounds can join chemically to form new compounds. During the process of photosynthesis plants take CO 2 and H 2 O molecules and use energy from sunlight to put them together to make C 6 H 12 O 6 molecules. CO 2 + H 2 O C 6 H 12 O 6
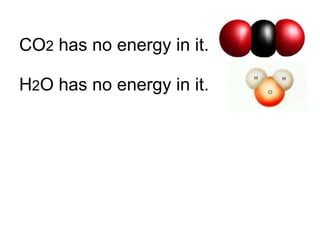
- 80. CO 2 has no energy in it. H 2 O has no energy in it. But C 6 H 12 O 6 (sugar) is the chemical energy that all living things must have to live and grow.
- 81. CO 2 has no energy in it. H 2 O has no energy in it. But C 6 H 12 O 6 (sugar) is the chemical energy that all living things must have to live and grow.
- 82. CO 2 has no energy in it. H 2 O has no energy in it. But C 6 H 12 O 6 (sugar) is the chemical energy that all living things must have to live and grow.
- 83. New compounds can have properties that are very different from properties of the elements or compounds that made them.
- 85. Atoms and molecules are way to small to see with your eye, or with a regular microscope. But scientists have recently discovered microscopes that can see them. Electron microscope
- 86. Atoms and molecules are way to small to see with your eye, or with a regular microscope. But scientists have recently discovered microscopes that can see them. Electron microscope Scanning tunneling microscope
- 87. The images from these instruments show that atoms and molecules are usually found in well ordered arrays , which means they are arranged neatly.
- 88. Atoms and molecules are found in well ordered arrays.
- 89. Atoms and molecules are found in well ordered arrays.
- 90. Atoms and molecules are found in well ordered arrays.
- 91. Atoms and molecules are found in well ordered arrays.
- 92. Atoms and molecules are found in well ordered arrays.
- 93. Atoms and molecules are found in well ordered arrays.
- 94. How are atoms arranged in images from advanced microscopes?
- 95. How are atoms arranged in images from advanced microscopes? Well ordered arrays
- 96. End of program
- 98. Cell diameter 15 microns