Rate and order of reaction
- 1. Rate and Order of Reaction Asad Bilal University Of Lahore Asad.bilal14@gmail.com 1
- 2. Agenda Concept of rate of reaction. Factors effecting rate of reaction. Concept of order of reaction. Methods for the determination of order of reaction. Pharmaceutical importance and applications of rate and order of reaction. 2
- 3. Reaction Rate What does “rate” mean ? Can you think of an everyday measurement of rate ? How about a car speed in miles per hour! How about water flow in gallons per minute! How about an audience entering a stadium in people per hour! 3
- 4. What do all these measures have in common??? 4
- 5. 5
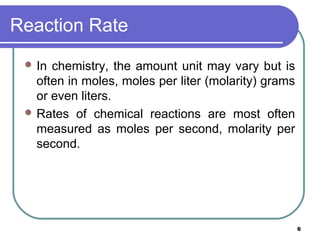
- 6. Reaction Rate In chemistry, the amount unit may vary but is often in moles, moles per liter (molarity) grams or even liters. Rates of chemical reactions are most often measured as moles per second, molarity per second. 6
- 8. Rate of Reaction Reaction rate is the speed at which a reaction takes place. It is “how quickly” a product is formed in a chemical reaction. Example Mg + Cl2 MgCl2 Reactants Product In the case of multiple step reactions the slowest step determines the rate of reaction. 8
- 10. Collision Theory Reactions take place when reactants bump to make products. Mg + Cl2 MgCl2 10
- 11. Collision Theory Reaction Rate is how quickly you create a new substance in a chemical reaction. Faster reactions have more collisions. Slower reactions have less collisions. 11
- 12. Dependence of Reaction Rate 12
- 13. Factors Effecting Rate of Reaction 13
- 14. Factors Effecting Rate of Reaction 14 Temperature Effect of concentration Light Solvent Ionic Strength Dielectric constant Catalysis
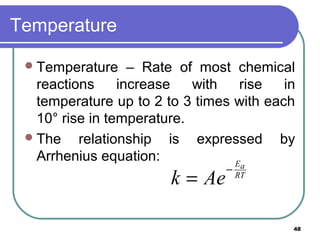
- 15. Temperature Generally, the speed of many reaction can be increased 2 to 3 times with each increase of 10o C in temperature. The effect of temperature on reaction rate is given by Arrhenius equation K= Ae-Ea/RT The frequency factor A is the measure of frequency expected between the reacting molecules. 15
- 16. In Logarithm it may be expressed as follow Log K= log A – Ea/ 2.303RT The Arrhenius equation is useful when Ea is in the range of 10 to 30 Kcal/mole. If Ea is only 2 to 3 Kcal/moleas in the case of photolytic reactions little advantage is gain from the equation. 16
- 17. Effect of Concentration As the concentration of reacting molecules is increased the no of collisions between the molecules also increased. Consequently the rate of reaction is increased. Concentration Collisions between molecules 17
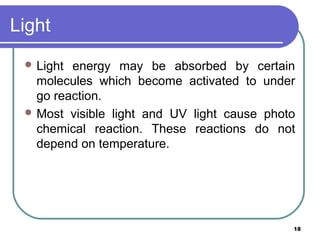
- 18. Light Light energy may be absorbed by certain molecules which become activated to under go reaction. Most visible light and UV light cause photo chemical reaction. These reactions do not depend on temperature. 18
- 19. However, Once a molecule have absorbed energy , It may collide with other molecules raising their kinetic energy resulting in increase in temperature. Examples: Pharmaceutical compounds which undergo photo chemical decomposition include Riboflavin and Phenothiazines etc. 19
- 20. Solvent The quantitative relationship between the reaction rate and the solubility of reactants and products is given by equation. Log k= log K0 + V/2.303 R . 1/T (∆SA+∆SB-∆S*) In other terms a polar solvent tends to increase the rate of those reactions in which product formed is more polar than reactants. 20
- 21. If the products are less polar then it tends to decrease the rate of such reactions. Commonly used non aqueous solvents for drugs include Ethanol, Glycerol and vegetable oil etc. 21
- 22. Ionic Strength The effect of ionic strength of a solutionand its rate of degradation may be expressed as follows Log K= log K0 + 1.02 ZAZB µѴ According to the above equation an increase in the ionic strength of solution would tend to decrease the rate of reaction. 22
- 23. Inverse trend Ionic Strength Rate of Reaction 23
- 24. Dielectric constant of solvent The dielectric constant (or relative permittivity ) of solvent has a significant effect on the rate of reaction. Dielectric constant of an ionic reaction is given by Log K= log K ε=∞ - K ZA ZB/ε 24
- 25. If the reacting ions are of opposite charges then it will result in increase rate of reaction. If ions of similar charges involve in reaction it will decrease rate of reaction. Increase in rate of reaction Opposite Charges Similar Charges 25
- 26. Catalysis A catalyst is defined as a substance which increase or decrease the rate of reaction without itself being altered chemically. Most of the chemical reactions are catalyzed in the presence of catalyst. These enhanced the rate of reaction by providing an alternative course for chemical reaction. 26
- 28. Order of Reaction The order of reaction is defined as the manner in which the rate of a reaction varies with the concentration of the reactants. 28
- 29. Types of Reactions With Respect to their Order Zero-Order Reaction First -Order Reaction Second-Order Reaction Pseudo-Zero-Order Reaction Pseudo-First-Order Reaction 29
- 30. Zero-Order Reaction In Zero-Order reaction the reaction rate is independent of the concentration of the reacting substance or reaction rate depends on the zero power of the reactant. Example Degradation of solution. When solubility is the factor , only that amount of drug that is in solution undergoes degradation. 30
- 31. First-Order Reaction A reaction is said to be first-order if the reaction rate depends on the first power of concentration of a single reactant. Example Decomposition of H2O2 catalyzed by iodine ions. 31
- 32. Second-Order Reaction A reaction is said to be second-order if the reaction rate depends on the concentration of two reactant species. Example Sponification of Ethyl acetate. 32
- 33. 33
- 34. Pseudo-Zero-Order Reaction Many drugs, in the solid state, decompose according to pseudo-zero- order rates as reactions occur between the drug and moisture in the solid dosage form. The system behaves as a suspension, and b/c of the presence of excess solid drug, the first-order reaction rate becomes a pseudo-zero-order rate, and loss rate is linear with time. 34
- 35. Equation 35
- 36. Pseudo-First-order Reaction A pseudo-first-order reaction can be defined as a second-order or bimolecular reaction that is made to behave like first-order reaction. This happens when one reacting material is present in great excess or is maintained at a constant concentration compared with the other substance. Under such circumstances the reaction does not exhibit a significant change in concentration during the degrative reaction. 36
- 37. Example Hydrolysis of an Ester. The drug that obeys pseudo-first- order kinetics is Cefotaxime sodium. 37
- 38. 38 Pharmaceutical Applications of Reaction Kinetics
- 39. 39 KINETICS Applications Chemical reactions such as decomposition of medicinal compounds Processes of drug absorption, distribution and elimination from the body Shelf life determination.
- 40. 40 Shelf life determination In determining the shelf life of a preparation, tests are carried out on the active ingredient, the additives and the finished product to determine: Whether decomposition will occur The type of decomposition Factors that affect the rate of decomposition such as light, air, moisture, temperature, etc. The influence of formulation additives The rate at which breakdown occurs.
- 41. 41 Order of Reaction Manner in which the rate of reaction varies with the concentration of the reactants Most processes involving ADME can be treated as first- order processes Some drug degradation processes can be treated as either First or zero order processes Some drug substances obey Michaelis- Menten kinetic process.
- 42. 42 Apparent Zero Order Reaction Kinetics Suspensions are a special case of zero order kinetics, in which the concentration of drug in solution depends on its solubility. As the drug in solution decomposes, more of it is released from a reservoir of suspended particles thereby making the concentration in solution constant. The effective concentration is the drug equilibrium solubility in the solvent of formulation at given temperatures
- 43. 43 Chemical instability Can present as; Loss of potency Accumulation of toxic degradative products Degrardation of excipient responsible for product stability e.g. emulsifying agents, preservatives Conspicuous colour change e.g. marked discoloration of adrenaline although very slight change in adrenaline content, is unacceptable to patients, pharmacists, physicians and the nurses.
- 44. 44 Solid state versus solution stability Generally, chemical reactions proceed more readily in liquid state than in solid state Serious stability problems are more commonly encountered in liquid medicines e.g. order of dosage form stability is generally: solution < suspension < tablet.
- 45. 45 Determination of Order of Reaction Use of rate equation – The data collected in a kinetic reaction should be substituted into the integrated form of equations of various orders. The process under test should be considered to be of that order where the calculated k value remains constant within limits of experimental error.
- 46. 46 Determination of Order of Reaction.. Half life method – For a zero order or pseudo first order reaction, t ½ is proportional to initial concentration of reactant (Co), t½ for a first order reaction is independent of Co, . Graphical method – For a zero order or pseudo first order reaction, plot of C vs. t is linear; for first order reaction, plot of log (Co- Ct) vs. t is linear.
- 47. 47 Factors Affecting Rate of Reactions The rate of reaction (degradation of pharmaceutical products) can be influenced temperature, moisture, solvent (pH, dielectric constant, etc), light (radiation), catalysts, oxygen and concentration of reactant (s).
- 48. 48 Temperature Temperature – Rate of most chemical reactions increase with rise in temperature up to 2 to 3 times with each 10° rise in temperature. The relationship is expressed by Arrhenius equation: RT aE Aek − =
- 49. 49 Activation Energy: Arrhenius Equation The degradation of a new cancer drug follows first-order kinetics and has degradation rate constants of 0.0001 H-1 at 60 ºC and 0.0009 H-1 at 80 ºC. What is its Ea?
- 50. 50 Stability Projection for Shelf Life The time required for 10 % of the drug to degrade with 90 % of intact drug remaining is based on Arrhenius equation: k = reaction rate, T = temperature, R = gas constant, Ea = activation energy 21 12 1 2 303.2 )( log TRT TTE k k a − =
- 51. 51 Concept of Q10 Q values of 2 (Ea ≈ 12.2 kcal/mole), 3 (Ea ≈ 19.4 kcal/mole), and 4 (Ea ≈24.5 kcal/mole) are commonly used They represent the energies of activation of the reactions around room temperature. T T K k Q )10( 10 + =
- 52. 52
- 53. 53 THANK YOU FOR YOUR ATTENTION