S4.4 Doubled Haploid Technology in Maize breeding: Status and prospects
- 1. Doubled Haploid Technology in Maize breeding: Status and prospects George Mahuku, Aida Kebede, Vanessa Prigge, Leocadio Martinez
- 2. Outline • Introduction to Doubled Haploid (DH) technology • Advantages of DH lines in maize breeding • Steps in DH line development • CIMMYT’s experience in DH line generation • Challenges • On-going activities
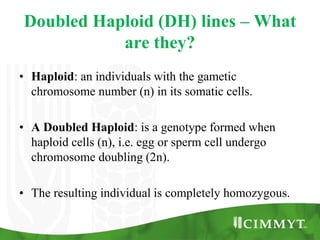
- 3. Doubled Haploid (DH) lines – What are they? • Haploid: an individuals with the gametic chromosome number (n) in its somatic cells. • A Doubled Haploid: is a genotype formed when haploid cells (n), i.e. egg or sperm cell undergo chromosome doubling (2n). • The resulting individual is completely homozygous.
- 4. Conventional vs DH Inbred Line Development • Produced by repeated generations of selfing • In each generation, heterozygosity reduces by 50% • Resulting inbred lines s are highly homozygous but not 100% • DH technique – a quicker method to obtain 100% pure inbred lines Generation S1 S2 S3 S4 S5 S6 S7 Homozygosity (%) 50 75 87.5 93.75 96.875 98.45 99.23 Months 6 12 18 24 30 36 42
- 5. Advantages of DH technique in hybrid maize breeding • Acceleration of inbred line development • Evaluation of putative hybrids at the beginning of the selection process • Maximum additive variance available • Reduction of masking effects which are caused by residual heterozygosity • Reduction of costs for nursery & maintenance breeding work • Simplyfied logistics Schmidt 2004; Röber et al. 2005
- 6. Doubled haploids – a valuable tool for research • Establishment of DH mapping populations – Improve the precision of genetic and mapping studies – Analysis of linkage disequilibrium – Analysis of haplotype/trait associations • Accelerate gene pyramiding • Evaluation, exploitation, and conservation of genetic resources – Extraction of individual gametes from heterozygous materials transforming them into DH lines – Detrimental effects are revealed to the full extent from the very beginning – Conservation of germplasm in form of reproducible DH lines
- 7. Methods for Producing haploids • In vitro - Tissue Culture Techniques – Anther Culture (microspore culture) – Highly complex & expensive – Low plantlet regeneration rate which is dependent on genetic background – Greatly limited for application in breeding programs • In vivo - Genetic induction – Widely used – Involves use of inducer lines – High frequency of haploid generation – Simple to operate – Relatively inexpensive
- 8. Two types of haploids Cytoplasm Chromosome Importance Effective for converting high Paternal Inducer Donor combining seed parent haploids lines to isogenic CMS analogues Rapid development of Maternal Donor Donor completely haploids homozygous inbred lines
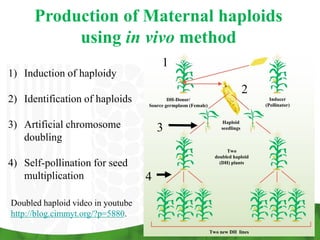
- 9. Production of Maternal haploids using in vivo method 1 1) Induction of haploidy 2 2) Identification of haploids DH-Donor/ Source germplasm (Female) Inducer (Pollinator) 3) Artificial chromosome 3 Haploid seedlings doubling Two doubled haploid 4) Self-pollination for seed (DH) plants multiplication 4 Doubled haploid video in youtube http://blog.cimmyt.org/?p=5880. Two new DH lines
- 10. Haploid Induction Table 1. Inducers and their haploid induction rate (HIR) Materials: Inducer HIR (%) Reference Stock 6 2.3 Coe 1959 Haploid inducer WS14 2.0 - 5.0 Lashermes & Beckert 1988 Heterozygous source KEMS RWS 6.3 8 - 23 Shatskaya et al. 1994 Röber et al. 2005 germplasm PK6 ~6 Barret et al. 2008 Prigge et al., in UH400 >8 preparation Collect inducer pollen Pollinate source germplasm R1-nj color marker
- 11. Induction in yellow Harvesting induced ears and white donors
- 12. Haploid Kernel Identification Triploid endosperm (purple aleurone) DH-Donor Inducer (purple embryo (colorless) & aleurone) X Diploid embryo (purple scutellum) R1-nj color marker system for identification of haploids (Coe and Sarkar, Haploid seed Regular (diploid) F1 - colorless embryo seed purple embryo 1964; Sarkar and Coe, - purple aleurone - purple aleurone 1966)
- 13. Haploid Kernel selection in CIMMYT Regular F1 Haploid CAT 1 (CAT 2) (CAT 3)
- 14. Step 3: Artificial genome doubling • 0.06% colchicine, 0.5% DMSO solution; 8 hours Gayen et al. (1994) MNL 68:85 • Colchicine acts as mitotic inhibitor Germination of haploid seeds Cutting of coleoptile on 3 consecutive days Colchicine treatment over night Transplanting to the field Planting into pots, recovery and establishing of treated plants
- 15. How does colchicine work? • Colchicine is an alhkloid produced by Colchicum autumnale • It works as mitotic inhibitor: by binding to tubulin during mitosis it inhibits spindle formation so that the cell cannot split into two daughter cells Haploid Doubled haploid (diploid)
- 16. Chromosome doubling agents • Colchicine is commonly used as doubling agent α and β Tubulin • Nitric Oxide (Kato and Geiger, 2002) • Microtubule binding herbicides • Caused chromosomal doubling of root tip cells (Hantzschel and Weber, 2010)
- 17. Step 4: Self-pollination Elimination of “false“plants • vigor & tillering • stalk color • endosperm & embryo color
- 18. DH lines express uniformity within the line and diversity among the lines! Cycle DH Conventional 1 Generate F1 Generate F1 2 Cross F1 x inducer Generate F2 3 Treat & self (D0) Generate F2:3 4 Self & generate Generate F3:4 D1 5 Generate F4:5 6 Generate F5:6
- 19. DH line generation at CIMMYT- progress
- 20. CIMMYT GMP started its involvement in DH in 2007 • University of Hohenheim provided temperate inducer genotypes and technical support • Various aspects under investigation: – Development of tropical adapted inducer lines – Induction rate of temperate inducers in tropical environments – Novel marker system for haploid kernel identification – Optimization of agronomic management to increase success rate of DH line development
- 21. Tropically adapted inducer line development New tropical Inducer lines Induction rate ≥10% Temperate inducer
- 22. Topically adapted Inducer lines TAIL Temperate Line Inducer
- 23. Progress in Haploid kernel induction Hand Pollination Isolation Block Hand Pollination Inducer Donor Inducer
- 24. Harvest from Isolation block Harvest of 2011 inductions •Moving into production phase •Increase the number of inductions to 150 source populations
- 25. Optimizing Agronomic Management
- 26. Lack of flowering or synchronization • Lack of synchronization • Good female flowers (stigmas) • Little or no pollen • Use of shading
- 27. Insect pest problem -Ear worms • Cipermetrina • Heliothis spp.
- 28. Mechanization
- 29. Progress: DH line development Goal : 5000 DH lines/year • 4350 DH lines generated in 2010/2011 • >10 000 DH lines in 2012 Cycle DH Conventional 1 Generate F1 Generate F1 2 Cross F1 x Generate F2 (LPS C7-F180-3-1-1-1-BBB / CML-449 ) inducer 3 Treat & self (D0) Generate F2:3 • Challenges • Agronomic management 4 Generate F3:4 • Haploid seed identification • Chromosome doubling 5 Generate F4:5 6 Generate F5:6
- 30. Number of lines 150 200 250 300 350 100 0 50 POP 1 POP 2 POP 3 POP 4 POP 5 POP 6 POP 7 POP 8 POP 9 POP 10 POP 11 POP 12 POP 13 POP 14 POP 15 POP 16 POP 17 POP 18 POP 19 POP 20 POP 21 POP 22 POP 23 POP 24 POP 25 POP 26 POP 27 POP 28 POP 29 POP 30 DH lines / population POP 31 POP 32 POP 33 POP 34 POP 35 POP 36 POP 37 POP 38 POP 39
- 31. D1 seeds per line 350 300 250 Number of lines 200 150 100 50 0 1 2 3 4 5 6 7 8 9 10 11 to 21 to 51 to100> 20 50 100 # Quantity of seed
- 32. On-going activities • Continue to optimize the DH production protocols • Develop a detailed protocol on how to develop DH lines • Finalize development of a tropically adapted inducer line • Look for new haploid seed identification phenotypic marker • Develop alternative chromosome doubling agents • Training partners in DH techniques
- 33. DH Group in Agua Fria