Sample prep
- 1. Sample preparation for cryo-EM Michael Landsberg School of Chemistry and Molecular Biosciences The University of Queensland m.landsberg@uq.edu.au Otago Cryo-EM Workshop January 2019
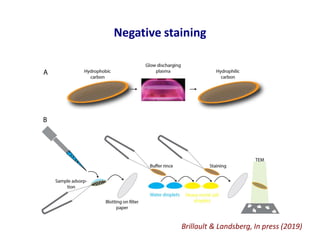
- 2. Negative staining Brillault & Landsberg, In press (2019)
- 3. Glow discharging • Glow discharging makes the carbon surface temporarily hydrophilic (negatively charged in air) • Altering the glow discharge parameters – Some hydrophobic proteins might not stick – Some proteins might stick too well • Glow discharge in a solvent atmosphere (e.g. amyl-amine) can alter the surface charge Brillault & Landsberg, In press (2019)
- 4. • Positively charged films required in order to get nucleic acids to stick
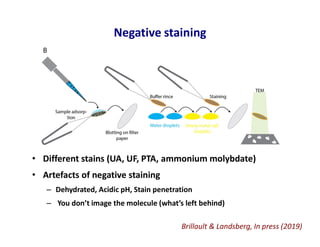
- 5. Negative staining • Different stains (UA, UF, PTA, ammonium molybdate) • Artefacts of negative staining – Dehydrated, Acidic pH, Stain penetration – You don’t image the molecule (what’s left behind) Brillault & Landsberg, In press (2019)
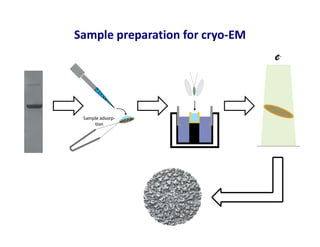
- 6. Sample preparation for cryo-EM e-
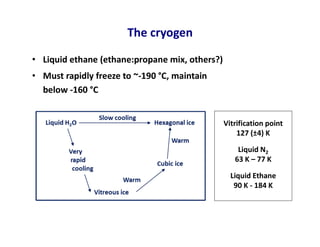
- 8. The cryogen • Liquid ethane (ethane:propane mix, others?) • Must rapidly freeze to ~-190 °C, maintain below -160 °C Vitrification point 127 (±4) K Liquid N2 63 K – 77 K Liquid Ethane 90 K - 184 K
- 11. Sample preparation is (and will likely remain) the rate limiting step
- 12. Sample preparation • The sample • The grid • The cryogen • Some problems you might encounter along the way
- 13. The sample • Highly pure (?) • “Crystallography grade” is good! • Historically big samples have been most successful but now samples as small as haemoglobin (64 kDa) have been reconstructed (with VPP)
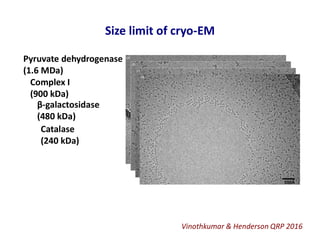
- 14. Size limit of cryo-EM Vinothkumar & Henderson QRP 2016 Pyruvate dehydrogenase (1.6 MDa) Complex I (900 kDa) β-galactosidase (480 kDa) Catalase (240 kDa)
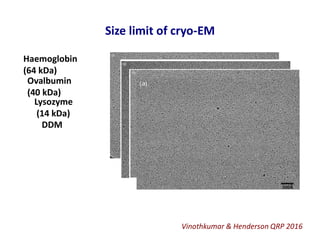
- 15. Size limit of cryo-EM Vinothkumar & Henderson QRP 2016 Haemoglobin (64 kDa) Ovalbumin (40 kDa) Lysozyme (14 kDa) DDM
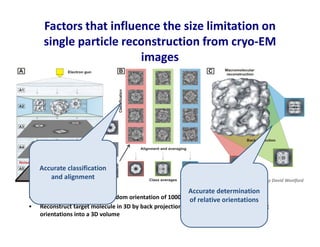
- 16. • Tilted views obtained by random orientation of 1000s of particles on an EM grid • Reconstruct target molecule in 3D by back projection of classes representing different orientations into a 3D volume Factors that influence the size limitation on single particle reconstruction from cryo-EM images Figure provided by David Woolford Accurate classification and alignment Accurate determination of relative orientations
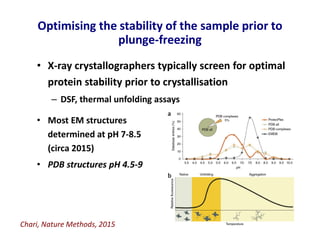
- 17. Optimising the stability of the sample prior to plunge-freezing • Most EM structures determined at pH 7-8.5 (circa 2015) • PDB structures pH 4.5-9 • X-ray crystallographers typically screen for optimal protein stability prior to crystallisation – DSF, thermal unfolding assays Chari, Nature Methods, 2015
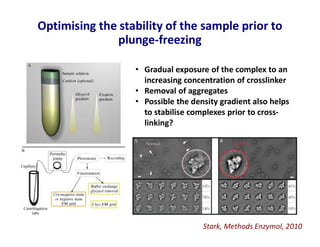
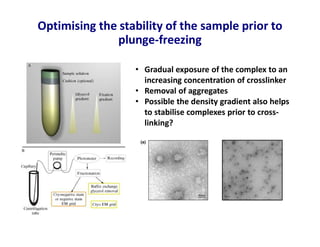
- 18. Optimising the stability of the sample prior to plunge-freezing Stark, Methods Enzymol, 2010 • Gradual exposure of the complex to an increasing concentration of crosslinker • Removal of aggregates • Possible the density gradient also helps to stabilise complexes prior to cross- linking?
- 19. Optimising the stability of the sample prior to plunge-freezing • Gradual exposure of the complex to an increasing concentration of crosslinker • Removal of aggregates • Possible the density gradient also helps to stabilise complexes prior to cross- linking?
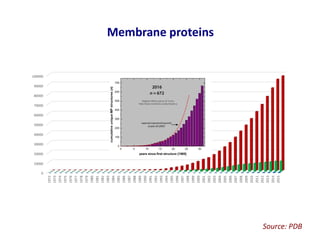
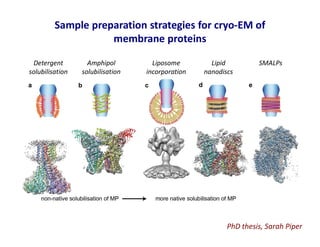
- 21. Sample preparation strategies for cryo-EM of membrane proteins Detergent solubilisation Amphipol solubilisation Liposome incorporation Lipid nanodiscs SMALPs PhD thesis, Sarah Piper
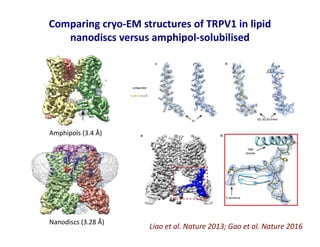
- 22. Comparing cryo-EM structures of TRPV1 in lipid nanodiscs versus amphipol-solubilised Liao et al. Nature 2013; Gao et al. Nature 2016 Amphipols (3.4 Å) Nanodiscs (3.28 Å)
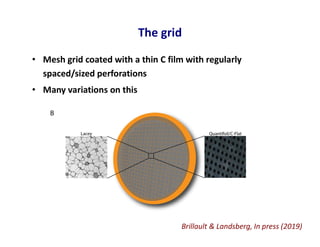
- 23. The grid • Mesh grid coated with a thin C film with regularly spaced/sized perforations • Many variations on this Brillault & Landsberg, In press (2019)
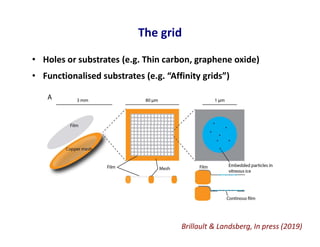
- 24. The grid • Holes or substrates (e.g. Thin carbon, graphene oxide) • Functionalised substrates (e.g. “Affinity grids”) Brillault & Landsberg, In press (2019)
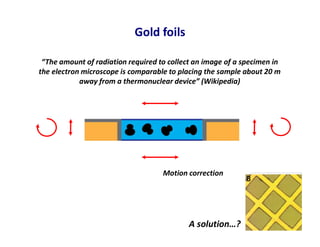
- 25. Gold foils “The amount of radiation required to collect an image of a specimen in the electron microscope is comparable to placing the sample about 20 m away from a thermonuclear device” (Wikipedia) Motion correction A solution…?
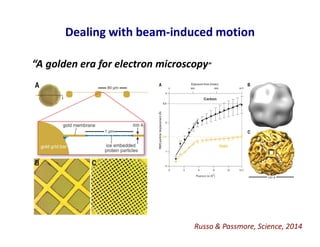
- 26. Dealing with beam-induced motion “A golden era for electron microscopy” Russo & Passmore, Science, 2014
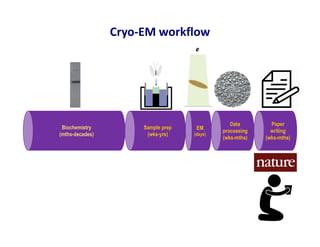
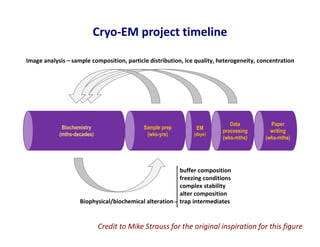
- 27. Cryo-EM project timeline Biochemistry (mths-decades) Sample prep (wks-yrs) EM (days) Data processing (wks-mths) Paper writing (wks-mths) buffer composition freezing conditions complex stability alter composition Biophysical/biochemical alteration trap intermediates Image analysis – sample composition, particle distribution, ice quality, heterogeneity, concentration Credit to Mike Strauss for the original inspiration for this figure
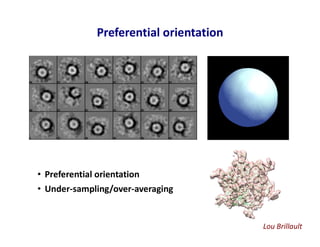
- 28. Preferential orientation • Preferential orientation • Under-sampling/over-averaging Lou Brillault
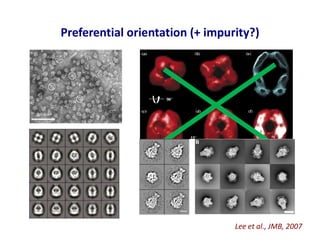
- 29. Preferential orientation (+ impurity?) Lee et al., JMB, 2007
- 30. Why do we get preferred orientations? Figure credit: Gavin Rice
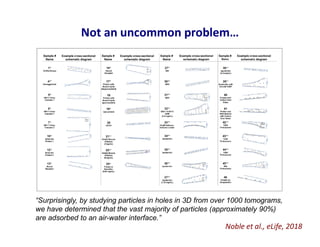
- 31. Not an uncommon problem… Noble et al., eLife, 2018 “Surprisingly, by studying particles in holes in 3D from over 1000 tomograms, we have determined that the vast majority of particles (approximately 90%) are adsorbed to an air-water interface.”
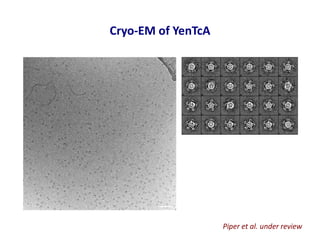
- 32. Cryo-EM of YenTcA Piper et al. under review
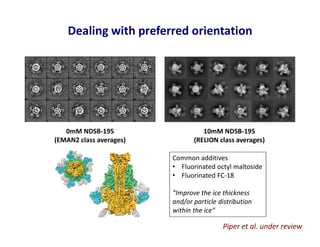
- 33. Dealing with preferred orientation 0mM NDSB-195 (EMAN2 class averages) 10mM NDSB-195 (RELION class averages) Piper et al. under review Common additives • Fluorinated octyl maltoside • Fluorinated FC-18 “Improve the ice thickness and/or particle distribution within the ice”
- 34. Approaches for dealing with the air-water interface Glaeser, COCIS, 2018
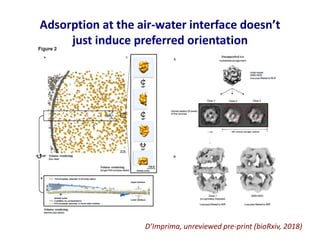
- 35. Adsorption at the air-water interface doesn’t just induce preferred orientation D’Imprima, unreviewed pre-print (bioRxiv, 2018)
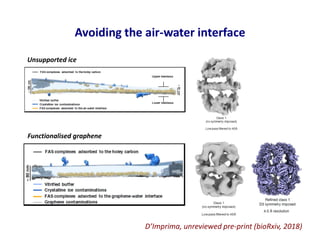
- 36. Avoiding the air-water interface D’Imprima, unreviewed pre-print (bioRxiv, 2018) Unsupported ice Functionalised graphene
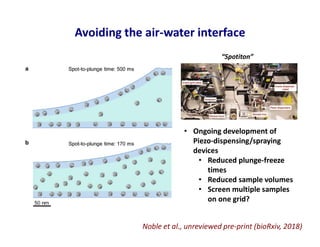
- 37. Avoiding the air-water interface Noble et al., unreviewed pre-print (bioRxiv, 2018) • Ongoing development of Piezo-dispensing/spraying devices • Reduced plunge-freeze times • Reduced sample volumes • Screen multiple samples on one grid? “Spotiton”
- 38. Summary • Cryo-EM sample preparation may not be as straightforward as the instruction manual suggests • Many opportunities for sample-dependent fine- tuning • Increasing automation (sample prep, imaging, image analysis) will undoubtedly help • Exciting ongoing developments and much scope to innovate further • Some samples just work!
- 39. Acknowledgements Lou Brillault Sarah Piper Gavin Rice Matthias Floetenmeyer Funding and infrastructure support Contributors