The mole
- 1. The Mole 6.02 X 10 23
- 2. The Mole • A counting unit • Similar to a dozen (12), except instead of 12, it’s 602 billion trillion 602,000,000,000,000,000,000,000 • 6.02 X 1023 (in scientific notation) • This number is named in honor of Amedeo Avagadro (1776 – 1856), who studied quantities of gases and discovered that no matter what the gas was, there were the same number of molecules present
- 3. Mole • A mole is just a number. Just like..... a pair = 2 a trio = 3 a quartet = 4 a dozen = 12 a mole = (mol) 602000000000000000000000
- 4. 4 Just How Big is a Mole? • Enough soft drink cans to cover the surface of the earth to a depth of over 200 miles. • If you had Avogadro's number of unpopped popcorn kernels, and spread them across the United States of America, the country would be covered in popcorn to a depth of over 9 miles. • If we were able to count atoms at the rate of 10 million per second, it would take about 2 billion years to count the atoms in one mole.
- 5. How Big is a Mole? One mole of marbles would cover the entire Earth (oceans included) for a depth of three miles. One mole of $100 bills stacked one on top of another would reach from the Sun to Pluto and back 7.5 million times. It would take light 9500 years to travel from the bottom to the top of a stack of 1 mole of $1 bills.
- 6. How BIG is a mole? There are ~ 6.6 billion people on Earth How many Earths would it take to equal the population of 1 mole? 9.12 x 1013
- 7. • If you had a mole of cats . . . They would create a sphere larger than Earth!
- 8. • If you had a mole of $$$$$ and you spent $800 billion dollars a day how many years would it take to spend a MOLEion dollars? 2.06 x 109 years
- 9. • If you had a mole of H2O could you swim in it? NO! Water molecules are so small that a mole of H2O = 18ml
- 10. How small are atoms? • There are more atoms in one gram of salt than grains of sand on all the beaches of all the oceans in all the world.
- 11. • Just one granule of sugar contains 1 x 10 17 molecules • Each time you take a breath of air, you inhale about 2 x 1022 molecules of nitrogen and 5 x 10 21 molecules of oxygen.
- 12. • In chemistry we don’t work with single atoms or molecules because they are too small to be weighed or measured • We have to work with LOTS of atoms in order to measure them THAT’s WHERE THE MOLE COMES IN!
- 14. Examining Molar Relationships in Balanced Equations 6 CO2 + 12 H2O → 6 O2 + C6H12O6 +6 H20 Balanced equations –Law of conservation of mass / matter • ATOMS are not created or destroyed during a chemical reaction, they are only rearranged to form new substances. • # atoms on reactant side = # atoms on product side – The Coefficient for each molecule tells you how many “moles” of the molecule are in the reaction. • For example: 6 CO2 = 6 moles of CO2
- 15. Learning Check #1 Suppose we invented a new unit called a rapp. One rapp contains 8 objects. 1. How many paper clips in 1 rapp? a) 1 b) 4 c) 8 2. How many oranges in 2.0 rapp? a) 4 b) 8 c) 16 3. How many rapps contain 40 gummy bears? a) 5 b) 10 c) 20
- 16. Learning Check #1 - Answers Suppose we invented a new unit called a rapp. One rapp contains 8 objects. 1. How many paper clips in 1 rapp? a) 1 b) 4 c) 8 2. How many oranges in 2.0 rapp? a) 4 b) 8 c) 16 3. How many rapps contain 40 gummy bears? a) 5 b) 10 c) 20
- 17. The Mole • 1 dozen cookies = 12 cookies • 1 mole of cookies = 6.02 X 1023 cookies • 1 dozen cars = 12 cars • 1 mole of cars = 6.02 X 1023 cars • 1 dozen Al atoms = 12 Al atoms • 1 mole of Al atoms = 6.02 X 1023 atoms Note that the NUMBER is always the same, but the MASS is very different! Mole is abbreviated mol (gee, that’s a lot quicker to write, huh?)
- 18. A Mole of Particles Contains 6.02 x 10 23 particles 1 mole C = 6.02 x 1023 C atoms 1 mole H2O = 6.02 x 10 23 H2O molecules 1 mole NaCl = 6.02 x 1023 NaCl “molecules” (technically, ions not molecules) 6.02 x 1023 Na + ions and 6.02 x 1023 Cl– ions Note that a particle could be an atom OR a molecule!
- 19. Avogadro’s Number as Conversion Factor 6.02 x 1023 particles 1 mole or 1 mole 6.02 x 1023 particles Note that a particle could be an atom OR a molecule!
- 20. Learning Check #2 1. Number of atoms in 1.0 mole of Al a) 500 Al atoms b) 6.02 x 1023 Al atoms c) 3.01 x 1023 Al atoms 2. Number of moles of S in 6.02 X 1023 S atoms a) 1.0 mole S atoms b) 6.02 mole S atoms c) 1 x 1023 mole S atoms
- 21. Learning Check #2 - Answers 1. Number of atoms in 1.0 mole of Al a) 500 Al atoms b) 6.02 x 1023 Al atoms c) 3.01 x 1023 Al atoms 2. Number of moles of S in 6.02 X 1023 S atoms a) 1.0 mole S atoms b) 6.02 mole S atoms c) 1 x 1023 mole S atoms
- 22. STOICHIOMETRY - the study of the quantitative (#) aspects of chemical reactions.
- 23. Molar Mass • The Mass of 1 mole in grams • Equal to the numerical value of the average atomic mass. This number is found on the periodic table, below the symbol for each element.
- 24. Molar Mass The Atomic Mass # does not have units in the periodic table. Often, chemists use “amu” for the atomic mass unit. To determine the molar mass, we change “amu” into “grams/mole” 1 amu = 1 gram check on your periodic table!!! 1 mole 1 mole of He atoms = 4.0 g 1 mole of C atoms = 12.0 g 1 mole of Mg atoms = 24.3 g 1 mole of Cu atoms = 63.5 g
- 25. Learning Check #3 Find the molar mass for the following elements: (round to the tenths place for decimals) A. 1 mole of Br atoms B. 1 mole of Sn atoms C. 2 moles of C atoms
- 26. Learning Check #3 - Answers Find the molar mass for the following elements: (round to the tenths place for decimals) A. 1 mole of Br atoms = 79.9 g/mol B. 1 mole of Sn atoms = 118.7 g/mol C. 2 moles of C atoms = 12 g/mole * 2 moles = 24 g/mol
- 27. Molar Mass of Molecules & Compounds ADD UP THE MASS FROM ALL ATOMS IN THE MOLECULE !!!!! ***The subscript tells you how many atoms for each element are present in the molecule 1 mole of the molecule CaCl2 = 111.1 g/mol HOW???? 1 mole Ca x 40.1 g/mol + 2 moles Cl x 35.5 g/mol = 111.1 g/mol CaCl
- 28. Learning Check #4 What is the Molar Mass of the molecule K2O? ****Units = g/mol
- 29. Learning Check #4 - Answers What is the Molar Mass of the molecule K2O? ****Units = g/mol 2 moles of K = 39 g/mol * 2 = 78 g/mol + 1 mole of O = 16 g/mol TOTAL = 94 g/mol !!!!
- 30. What is Dimensional Analysis? Dimensional analysis is just a fancy name for a method of calculating that: 1. uses numbers in the form of fractions. 2. lets us convert from one type of unit measurement to another. 3. In chemistry, this lets us convert from moles to grams or visa-versa
- 31. What is a Unit? A unit is something that gives definition to a numerical value, quantity, or measurement. • Length : meters, centimeters, feet, inches, miles, kilometers • Mass : Kilograms, grams, pounds • Time : hours, minutes, seconds, days, months • Volume : cups, teaspoons, liters, milliliters, gallons, quarts • Currency: dollars, cents, dimes
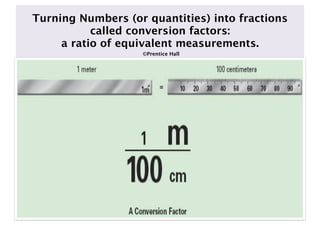
- 32. Turning Numbers (or quantities) into fractions called conversion factors: a ratio of equivalent measurements. ©Prentice Hall
- 33. Conversion Factors Conversion factors can be written in 2 ways: The unit you want goes on top or the numerator and the unit you want to cancel goes on the bottom or denominator.
- 34. Converting Moles and Grams Aluminum is often used for the structure of light-weight bicycle frames. How many grams of Al are in 3.00 moles of Al? 3.00 moles Al ? g Al
- 35. 1. Molar mass of Al 1 mole Al = 27.0 g Al 2. Conversion factors for Al 27.0 g Al or 1 mol Al 1 mol Al 27.0 g Al Why? Because I want to “cancel” mol and be left with g. g must be on top. 3. Setup 3.00 moles Al x 27.0 g Al 1 mole Al 4. Math 3 * 27/1 = 81 5. Answer = 81.0 g Al