The Nuclear Atom
- 1. { THE NUCLEAR ATOM ATOMIC MODEL NUCLUES
- 2. {Describe the structure of the atom in terms of nucleus and electrons. Atomic Model
- 3. In physics, an atom (Greek ‘átomos’ meaning "indivisible") is the smallest particle which characterise a chemical element. The atom is composed of subatomic particles: electrons; protons; neutrons. Protons and neutrons make up a dense, massive atomic nucleus, and are collectively called nucleons. The electrons form the much larger electron cloud surrounding the nucleus. Atom
- 5. {Describe how the Geiger-Marsden alpha- particle scattering experiment provides evidence for the nuclear atom. Atomic Model
- 6. The plum pudding model of the atom was proposed by J. J. Thomson, the discoverer of the electron in 1896. In this model, the atom is composed of electrons surrounded by a soup of positive charge to balance the electron's negative charge, like plums surrounded by pudding. Plum Pudding Model
- 7. This is also called the Gold foil experiment or the Rutherford experiment was an experiment done by Hans Geiger and Ernest Marsden in 1909 which led to the downfall of the plum pudding model of the atom. They observed that a very small percentage of particles were deflected through angles much larger than 90 degrees; some were even scattered back toward the source. Geiger-Marsden Experiment
- 8. Top: Expected results of Rutherford's gold foil experiment: alpha particles passing through the plum pudding model of the atom undisturbed. Bottom: Observed results: Some of the particles were deflected, and some by very large angles. Rutherford concluded that the positive charge of the atom must be concentrated into a very small location: the atomic nucleus.
- 9. Atom Models in History
- 10. {Describe the composition of the nucleus in terms of protons and neutrons. Nucleus
- 11. {Define the terms proton number (atomic number), Z and nucleon number (mass number), A. Nucleus
- 12. {Explain the term nuclide. Nucleus
- 13. Nuclei are made up of positive protons and neutral neutrons bound together by the strong force. Both protons and neutrons are referred to as nucleons. The number of protons in the nucleus is called the atomic number Z The total number of neutrons and protons is called the mass number A. Nucleus
- 14. Nuclear Notation
- 15. {Define the term isotope. Nucleus
- 16. {Explain, using nuclide notation, how one element may have a number of isotopes. Nucleus
- 17. The different isotopes of a given element have the same atomic number but different mass numbers since they have different numbers of neutrons. The chemical properties of the different isotopes of an element are identical, but they will often have great differences in nuclear stability. Isotopes
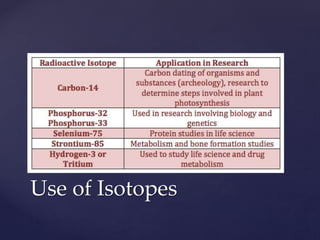
- 19. Use of Isotopes
- 20. Use of Isotopes
- 21. Use of Isotopes
- 23. 1. In the atomic model, an atom consists of a central mass, orbited by much smaller particles.
- 24. 1. What is the name of the central mass and of the orbiting particles? D
- 25. 2. Between 1909 and 1911, Geiger and Marsden carried out experiments in which alpha particles were fired at metal foil. Most of the alpha particles passed through the foil with small deflections, but some were deflected through a large angle. 1. These results suggest that A. atoms contain clouds of electrons through which some alpha particles cannot pass. B. atoms contain neutrons that alpha particles bounce off. C. atoms have positive and negative charges spread throughout their volume. D. atoms have positive charges concentrated in a small volume.
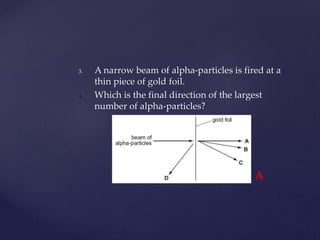
- 26. 3. A narrow beam of alpha-particles is fired at a thin piece of gold foil. 4. Which is the final direction of the largest number of alpha-particles? A
- 27. 4. Which conclusion can be drawn from the Geiger-Marsden alpha-particle scattering experiment? A. A positive charge is spread throughout the atom. B. Electrons are arranged in orbits. C. Electrons are negatively charged. D. There is a dense nucleus in the atom.
- 28. 5. is the symbol for a particular nuclide of nitrogen. 6. How many nucleons does this nuclide contain? A. 7 B. 9 C. 16 D. 23
- 29. 6. An atom of the element lithium has a nucleon number of 7 and a proton number of 3. 7. Which diagram represents a neutral atom of lithium? C
- 30. 7. The data below relates to the nucleus of a particular neutral atom of nitrogen. proton number Z = 7 nucleon number A = 17 1. Which row represents the correct number of neutrons and electrons in this atom? A
- 31. 8. A nuclide of strontium is represented by the symbol 9. What does the nucleus contain? A. 38 electrons and 50 neutrons B. 38 neutrons and 38 protons C. 38 neutrons and 50 protons D. 38 protons and 50 neutrons
- 32. 9. A nucleus of the element cobalt may be represented by the symbol . 10. What is the structure of this nucleus? A
- 33. 10. How many neutrons and how many protons are contained in a nucleus of ? B
- 34. 11. What are the numbers of neutrons, protons and electrons in a neutral atom of ? C
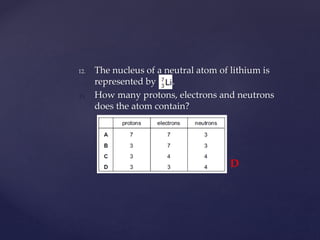
- 35. 12. The nucleus of a neutral atom of lithium is represented by . 13. How many protons, electrons and neutrons does the atom contain? D
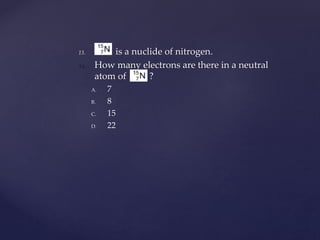
- 36. 13. is a nuclide of nitrogen. 14. How many electrons are there in a neutral atom of ? A. 7 B. 8 C. 15 D. 22
- 37. 14. The nuclide notation for radium-226 is . 15. How many electrons orbit the nucleus of a neutral atom of radium-226? A. 0 B. 88 C. 138 D. 226
- 38. 15. A uranium nucleus emits an α-particle. 16. What are the new nucleon and proton numbers? D
- 39. 16. A particular nuclide has the symbol . 17. What is true for atoms of this nuclide? A. There are 17 nucleons in the nucleus. B. There are 17 protons in the nucleus. C. There are 37 electrons in the nucleus. D. There are 37 neutrons in the nucleus.
- 40. 17. Proton number is another name for atomic number. Nucleon number is another name for mass number. 18. What are isotopes? A. nuclei with different proton numbers and different nucleon numbers B. nuclei with different proton numbers and the same nucleon number C. nuclei with the same proton number and different nucleon numbers D. nuclei with the same proton number and the same nucleon number
- 41. 18. The neutral atoms of all isotopes of the same element contain the same number of A. electrons and protons. B. electrons and neutrons. C. neutrons only. D. neutrons and protons.
- 42. 19. A nuclide has the notation . 20. Which line in the table describes a different isotope of this nuclide? A
- 43. 20. There are three nuclides of hydrogen.
- 44. 1. Which of these nuclides have the same number of protons in their nuclei? A. 1 and 2 only B. 2 and 3 only C. all of them D. none of them