TOPIC 4 LIPID METABOLISM.pptx
- 1. TOPIC 4 LIPIDS METABOLISM BASIC CHEMISTRY AF 1082
- 2. LEARNING OUTCOME AT THE END OF THIS TOPIC, STUDENT WILL BE ABLE TO: • EXPLAIN LIPIDS AND IDENTIFY THE CHARACTERISTICS OF LIPIDS • IDENTIFY AND DESCRIBE DIFFERENT CLASSIFICATION OF LIPIDS • DESCRIBE THE METABOLIC PATHWAY OF LIPIDS • EXPLAIN THE METABOLIC DISORDER OF LIPIDS: DIABETIC KETOACIDOSIS, CARDIOVASCULAR DISEASE
- 3. WHAT ARE LIPIDS? • LIPIDS ARE ORGANIC COMPOUNDS THAT CONTAIN HYDROGEN, CARBON, AND OXYGEN ATOMS, FORMING NONPOLAR MOLECULES THAT ARE SOLUBLE IN NONPOLAR SOLVENT AND INSOLUBLE IN POLAR SOLVENT SUCH AS WATER. • LIPIDS ARE NOT DEFINED BY THE PRESENCE OF SPECIFIC FUNCTIONAL GROUP LIKE CARBOHYDRATE, BUT BY PHYSICAL PROPERTY – SOLUBILITY. • LIPIDS ARE FATTY, WAXY, OR OILY COMPOUNDS. LIPIDS INCLUDE: • FATS AND OILS (TRIGLYCERIDES) • PHOSPHOLIPIDS • WAXES • STEROIDS
- 4. CHARACTERISTICS OF LIPIDS • ENERGY-RICH ORGANIC MOLECULES • INSOLUBLE IN WATER • SOLUBLE IN ORGANIC SOLVENT LIKE ALCOHOL, CHLOROFORM, ACETONE, BENZENE ETC • ON HYDROLYSIS THEY GIVE FATTY ACIDS • SOLID TRIGLYCEROLS (FATS) HAVE HIGH PROPORTIONS OF SATURATED FATTY ACIDS • LIQUID TRIGLYCEROLS (OILS) HAVE HIGH PROPORTIONS OF UNSATURATED FATTY ACIDS
- 5. FUNCTIONS OF LIPIDS • ENERGY STORAGE, MOBILIZATION, AND UTILIZATION • CELL DIFFERENTIATION AND GROWTH • CELL MEMBRANE STRUCTURE • SIGNAL TRANSMISSION • HORMONE SYNTHESIS • BILE ACIDS SYNTHESIS
- 6. CLASSIFICATION OF LIPIDS LIPIDS SIMPLE LIPIDS Esters of fatty acids with various alcohols FATS (TRIGLYCERIDES) Esters of fatty acids with glycerol. Oils are fats in the liquid state WAXES Esters of fatty acids with higher molecular weight monohydric alcohols COMPLEX LIPIDS Esters of fatty acids containing groups in addition to alcohol and fatty acids PHOSPHOLIPIDS This lipids contain additional phosphate group. Frequently have nitrogen-containing base and other substituent. GLYCOLIPIDS Containing fatty acids, sphingosine and carbohydrate LIPOPROTEIN Containing lipid and protein PRECURSOR AND DERIVED LIPIDS These compounds are produces by hydrolysis of simple and complex lipids STEROIDS CHOLESTEROLS SEX HORMONES
- 7. FATTY ACIDS
- 8. WHAT IS FATTY ACIDS? • COMMON FEATURES OF LIPIDS IS THAT THEY ARE ALL ESTERS OF MODERATE TO LONG CHAIN OF FATTY ACIDS AS IT IS THE SIMPLEST FROM OF LIPID. • FATTY ACIDS IS A CARBOXYLIC ACID WITH A LONG SIDE CHAIN OF HYDROCARBON • IT IS PRODUCED BY THE BREAKDOWN OF FATS (USUALLY TRIGLYCERIDES OR PHOSPHOLIPIDS) THROUGH A PROCESS CALLED HYDROLYSIS. • FATTY ACID MAY BE PRESENTED BY R-COOH, WHERE R STANDS FOR THE ALIPHATIC MOIETY AND COOH AS THE CARBOXYLIC GROUP Aliphatic: compound composed of carbon and hydrogen arranged in straight or branched chains, not containing rings
- 9. CLASSIFICATION OF FATTY ACIDS • FATTY ACIDS CAN BE CLASSIFIED BY THE PRESENCE AND NUMBER OF CARBON- TO-CARBON DOUBLE BOND THERE ARE THREE TWO TYPES FATTY ACIDS: 1. SATURATED FATTY ACIDS: ALL CARBON ATOMS IN THE CHAIN ARE SINGLE BONDS, AND REMAINING BONDS ARE ATTACHED TO HYDROGEN 2. NON SATURATED FATTY ACIDS: CONTAIN ONE OR MORE CARBON-TO-CARBON DOUBLE BONDS Fatty Acids Saturated Unsaturated 1 2 1
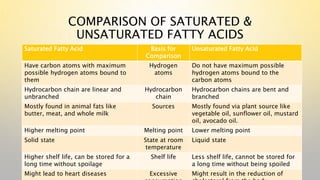
- 10. COMPARISON OF SATURATED & UNSATURATED FATTY ACIDS Saturated Fatty Acid Basis for Comparison Unsaturated Fatty Acid Have carbon atoms with maximum possible hydrogen atoms bound to them Hydrogen atoms Do not have maximum possible hydrogen atoms bound to the carbon atoms Hydrocarbon chain are linear and unbranched Hydrocarbon chain Hydrocarbon chains are bent and branched Mostly found in animal fats like butter, meat, and whole milk Sources Mostly found via plant source like vegetable oil, sunflower oil, mustard oil, avocado oil. Higher melting point Melting point Lower melting point Solid state State at room temperature Liquid state Higher shelf life, can be stored for a long time without spoilage Shelf life Less shelf life, cannot be stored for a long time without being spoiled Might lead to heart diseases Excessive Might result in the reduction of
- 11. SIMPLE LIPIDS 1. TRIGLYCERIDES (FATS AND OILS)
- 12. TRIGLYCERIDES (FATS AND OILS) • ESTERS MADE UP OF GLYCEROL AND 3 FATTY ACIDS. • TRIACYLGLYCEROL IS THE CORRECT CHEMICAL NAME, BUT COMMONLY KNOWN AS TRIGLYCERIDE. • MAIN STORAGE OF FATTY ACIDS • MAJOR DIETARY FAT
- 13. PHYSICAL PROPERTIES OF FATS AND OILS • COLOURLESS, ODOURLESS AND TASTELESS • LIGHTER THAN WATER, DENSITIES OF ABOUT 0.8G/CM3 • POOR CONDUCTORS OF HEAT AND ELECTRICITY>>EXCELLENT INSULATOR FOR THE BODY, SLOWING DOWN THE LOSS OF HEAT THROUGH THE SKIN • FAT IS SOLID AT 25OC, CONTAIN HIGH PROPORTION OF SATURATED FATTY ACIDS • OIL IS LIQUID AT 25OC, CONTAINS HIGH PROPORTION OF UNSATURATED FATTY ACIDS
- 14. FUNCTIONS OF FATS IN THE BODY 1. ENERGY SOURCE: FATS PRODUCE MORE THAN DOUBLE THE ENERGY PRODUCED BY PROTEINS AND CARBOHYDRATES 2. STORAGE: A MEAN TO TORE FOOD IN THE BODY FOR ENERGY AND TO PROTECT INTERNAL ORGANS 3. KEEPS BODY WARMS IN COLD WEATHER 4. PRESENT IN CELL STRUCTURE AND NERVE TISSUES
- 16. WAXES • CONSIST OF LONG CHAIN FATTY ACIDS LINKED THROUGH ESTER OXYGEN TO A LONG-CHAIN ALCOHOL • COMPLETELY WATER INSOLUBLE AND GENERALLY SOLID AT BIOLOGICAL TEMPERATURES. • STRONG HYDROPHOBIC NATURE, ALLOWS THEM TO FUNCTION AS WATER REPELLENTS • NATURALLY FOUND ON PLANTS AS A PROTECTIVE COATING TO CONTROL EVAPORATION AND HYDRATION. • BEST KNOWN ANIMAL WAX IS BEESWAX, WHICH BEES USE FOR CONSTRUCTING HONEYCOMB • SYNTHETIC AND WAXES ARE USED IN ADHESIVES, COSMETICS, FOOD, CANDLES AND MANY OTHER COMMERCIAL PRODUCTS
- 18. PHOSPHOLIPIDS • MAJOR COMPONENTS OF THE PLASMA MEMBRANE, THE OUTERMOST LAYER OF ANIMAL CELLS. • PHOSPHOLIPID MOLECULE: A MOLECULE WITH 2 FATTY ACIDS AND A MODIFIED PHOSPHATE GROUP ATTACHED TO GLYCEROL BACKBONE. • PHOSPHOLIPID MOLECULE IS AN AMPHIPATHIC MOLECULE WHICH MEANS IT HAS BOTH HYDROPHOBIC AND HYDROPHILIC COMPONENT • IN CELL MEMBRANE, PHOSPHOLIPIDS ARE ARRANGED IN A BILAYER MANNER, PROVIDING CELL PROTECTION AND SERVING
- 19. FUNCTIONS OF PHOSPHOLIPIDS • AS MEMBRANE COMPONENT, PHOSPHOLIPIDS ARE SEMI-PERMEABLE, ALLOWS ONLY CERTAIN MOLECULES PASS THROUGH THEM TO ENTER THE CELLS. MOLECULES THAT ARE NONPOLAR OR SMALL POLAR MOLECULES SUCH AS O2, CO2, AND UREA. LARGE, POLAR MOLECULES LIKE GLUCOSE OR ION LIKE SODIUM AND POTASSIUM CANNOT PASS THROUGH EASILY. THIS HELPS KEEPS THE CONTENT OF THE CELL WORKING PROPERLY AND SEPARATE THE INSIDE OF THE CELL FROM SURROUNDING ENVIRONMENT. • PHOSPHOLIPIDS CAN BE BROKEN DOWN IN THE CELL AND USED FOR ENERGY • FOUND IN LUNG AND JOINTS WHERE THEY HELP LUBRICATE CELLS. • IN PHARMACEUTICALS, IT IS USED AS PART OF DRUG DELIVERY SYSTEMS, IT HELPS TRANSPORT S DRUG THROUGHOUT THE BODY TO THE AREA THAT IT IS MEANT TO AFFECT. EG: VALIUM • IN FOOD INDUSTRIES, PHOSPHOLIPIDS ACT AS EMULSIFIER, WHICH SUBSTANCE THAT DISPERSE OILS DROPLET IN WATER SO THAT THE OIL AND WATER DO NOT FORM SEPARATE LAYER. EG: EGG YOLKS CONTAIN PHOSPHOLIPIDS, USED IN MAYONNAISE
- 21. GLYCOLIPIDS • TYPE OF COMPLEX LIPIDS COMPRISING CARBOHYDRATE, FATTY ACIDS, SPHINGOLIPIDS OR A GLYCEROL GROUP • THESE MOLECULES ARE WIDELY DISTRIBUTED IN TISSUE, BRAIN AND ALSO NERVE CELLS
- 22. TWO MAIN CLASSES OF GLYCOLIPIDS: 1. GLYCOSPHINGOLIPIDS: A TYPE OF GLYCOLIPID CONTAINING SPHINGOSINE, FATTY ACIDS AND CARBOHYDRATE SPHINGOMYELIN: AN INSULATOR IN MYELIN SHEATH OF NERVE CELLS CEREBROSIDES: OFTEN FOUND WITHIN BRAIN TISSUE 2. GLYCOGLYCEROLIPIDS: A TYPE OF GLYCOLIPID CONTAINING GLYCEROL, FATTY ACIDS AND CARBOHYDRATE GLYCOPHOSPHOLIPIDS: FOUND IN RED BLOOD CELLS SULFOGLYCOGLYEROLIPIDS: LOCATED IN THE PHOTOSYNTHETIC CELLS OF PLANTS
- 23. FUNCTION OF GLYCOLIPIDS • PROVIDE ENERGY TO CELLS • ESSENTIAL PART OF CELL MEMBRANE • HELPS DETERMINING BLOOD GROUP OF AN INDIVIDUAL • ACT AS RECEPTORS AT THE SURFACE OF RED BLOOD CELLS • IT ALSO FUNCTIONS BY ASSISTING IMMUNE SYSTEM BY DESTROYING AND ELIMINATING PATHOGEN FROM BODY
- 25. LIPOPROTEIN • LIPIDS THAT CONTAIN PROTEINS • BINDING OF THE PROTEIN ALLOWS THE FATS TO MOVE THROUGH THE WATER INSIDE AND OUTSIDE CELLS
- 26. CLASSIFICATION OF LIPOPROTEINS LIPOPROTEIN CLASSIFICATIO N Cholesterol composition Triglycerides composition Functions High density lipoprotein (HDL) High Lowest Functions to deliver cholesterol from extrahepatic tissue to the liver for elimination Low density lipoprotein (LDL) Highest Low Functions to deliver cholesterol to the peripheral tissues and to liver Very low density lipoprotein (VLDL) Low High Functions to deliver triglycerides from liver to peripheral tissues Chylomicrons Lowest Highest Functions to deliver dietary triglycerides to peripheral tissues
- 27. PRECURSOR AND DERIVED LIPIDS CHOLESTEROL
- 28. CHOLESTEROL • MOST ABUNDANT STEROID IN THE HUMAN BODY • CHOLESTEROL IS THE WAX-LIKE SUBSTANCE, AN IMPORTANT LIPID FOUND IN THE CELL MEMBRANE. IN HUMAN BODY, CHOLESTEROL IS SYNTHESIZED IN THE LIVER. • FUNCTION: • MAJOR COMPONENT OF CELL MEMBRANE (ESPECIALLY ABUNDANT IN NERVE AND BRAIN TISSUE) • IMPORTANT IN THE SYNTHESIS OF BILE ACIDS. BILE ACID IS ESSENTIAL FOR DIGESTION OF FAT • PRECURSOR OF ALL STEROID HORMONES, NAMELY ANDROGENS, ESTROGENS, PROGESTINS,
- 30. 1. DIGESTION AND ABSORPTION OF LIPID 1. LIPID DIGESTED IN THE FORM OF TRIGLYCERIDE 2. DIETARY TRIGLYCERIDE BROKEN DOWN BEFORE BEING ABSORBED BY THE INTESTINE 3. BILE SALT SECRETED BY LIVER, STORED IN GALLBLADDER ARE RELEASED INTO SMALL INTESTINE TO SOLUBILIZE THE TRIGLYCERIDES 4. PANCREATIC LIPASE IS PRODUCED BY PANCREAS AND RELEASED INTO SMALL INTESTINE TO BREAK DOWN TRIGLYCERIDES INTO MONOGLYCERIDES, FATTY ACIDS AND GLYCEROL 5. IN THE INTESTINAL MUCOSAL CELLS, THE FATTY ACIDS AND MONOGLYCERIDES ARE RESYNTHESIZED INTO TRIGLYCERIDES AND PACKAGED INTO CHYLOMICRONS WHICH THEN ENTERS THE LYMPH VESSEL. 6. CHYLOMICRONS ENABLE FATS AND CHOLESTEROL TO MOVE WITHIN AQUEOUS ENVIRONMENT OF LYMPHATIC AND CIRCULATORY SYSTEM. 7. THEY CAN GO TO LIVER OR BEING STORED IN FAT CELLS (ADIPOCYTES) THAT COMPRISE ADIPOSE TISSUE FOUND
- 31. 2.A) LIPOLYSIS • TO OBTAIN ENERGY, TRIGLYCERIDES ARE BROKEN DOWN BY HYDROLYSIS INTO FATTY ACIDS AND GLYCEROL. THIS PROCESS IS CALLED LIPOLYSIS • LIPOLYSIS PROCESS TAKES PLACE IN CYTOPLASM Triglycerides from adipocytes Fatty Acid Glycerol lipolysis FA undergo oxidation by β-oxidation into acetyl CoA which is use in TCA cycle Glycerol Glycerol-3-Phosphate dihydroxyacetone phosph ATP ADP NAD+ NADH + H+ gluconeogenesis Glycerol kinase Glycerol phosphate dehydrogena se
- 32. 3.A) FATTY ACIDS CATABOLISM 1. BREAKDOWN OF FATTY ACIDS (BETA OXIDATION) • DEGRADATION OF FATTY ACIDS BY REMOVING TWO CARBONS AT A TIME. • TAKES PLACE IN MITOCHONDRIAL MATRIX. • BETA OXIDATION END PRODUCT: • NADH • FADH • ACETYL COA WHICH IS USE IN TCA CYCLE TO MAKE ATP
- 33. 4.A) KETOGENESIS • IF EXCESS ACETYL COA CREATED, AND OVERLOADS CAPACITY OF TCA CYCLE, THE ACETYL COA CAN BE USED TO SYNTHESIZE KETONE BODIES. • KETOGENESIS HAPPENS IN THE MITOCHONDRIA • KETONE BODIES WILL BE USED BY THE HEART AND SKELETAL MUSCLES TO PRESERVE THE LIMITED GLUCOSE LEVEL DURING STARVATION. • 3 MAJOR TYPES OF KETONES: 1. ACETOACETATE 2. ACETONE 3. ΒETA-HYDROXYBUTYRATE. • KETONES HAVE A CHARACTERISTIC FRUITY SMELL.
- 34. 5.A) UTILIZATION OF KETONE BODIES • ACETONE IS NOT PRODUCTIVE MOLECULE, AND EXPELLED THROUGH THE LUNGS. • ACETOACETATE AND BETA-HYDROXYBUTYRATE ARE WATER-SOLUBLE, ABLE TO TRAVEL FREELY THROUGH BLOOD TO THE EXTRAHEPATIC TISSUES. • THEY ARE CONVERTED BACK TO ACETYL-COA WHEN IN NEEDS AND ENTERS THE TCA CYCLE. ADDITIONAL KETONE BODIES EXCRETED BY URINE.
- 35. 2.B) BLOOD CHOLESTEROLS FORMATION • AFTER CIRCULATING THROUGHOUT THE BODY, CHYLOMICRONS GRADUALLY RELEASE THEIR TRIGLYCERIDES UNTIL ALL THAT IS LEFT OF THEIR COMPOSITION IS CHOLESTEROL-RICH REMNANTS. • THIS REMNANTS USED BY THE LIVER TO FORMULATE SPECIFIC LIPOPROTEINS. i. VLDLS: TRANSPORT TRIGLYCERIDES FROM LIVER TO VARIOUS TISSUES IN BODY. AS VLDL TRAVELS, THE LIPOPROTEIN LIPASE REMOVE TRIGLYCERIDES FROM VLDL. VLDL NOW BECOME IDL. ii. IDL: WHILE TRAVELLING, CHOLESTEROL IS GAINED FROM OTHER LIPOPROTEINS. WHILE CIRCULATING ENZYMES REMOVES ITS PHOSPHOLIPID COMPONENT. IDL RETURN TO LIVER AND TRANSFORMED INTO LDL. iii. LDL: “BAD CHOLESTEROL”. LDL DELIVER CHOLESTEROL AND OTHER LIPIDS TO CELLS, EACH CELL’S SURFACE HAS RECEPTOR SYSTEMS SPECIFICALLY DESIGNED TO BIND WITH LDL. ONCE INSIDE THE CELL, LDL TAKEN APART AND ITS CHOLESTEROLS IS RELEASED. IN LIVER CELLS, THESE RECEPTOR SYSTEMS AID IN CONTROLLING BLOOD CHOLESTEROL LEVELS AS THEY BIND THE LDL. iv. HDL: “GOOD CHOLESTEROL” RESPONSIBLE FOR CARRYING CHOLESTEROL OUT OF THE BLOOD-
- 36. SUMMARY OF LIPID METABOLISM DIGESTION OF LIPID AND FORM CHYLOMICRONS LYMPH VESSEL CIRCULATORY SYSTEM ADIPOSE TISSUE GLYCEROL LIPOLYSIS GLUCONEOGENESIS BLOOD CHOLESTEROLS FORMATION UTILIZATION OF KETONE KETOGENESIS FATTY ACIDS CATABOLISM FATTY ACIDS
- 37. LIPID METABOLISM DISORDER 1. DIABETIC KETOACIDOSIS 2. CARDIOVASCULAR DISEASE
- 39. 1. DIABETIC KETOACIDOSIS • DIABETES KETOACIDOSIS (DKA) IS A CONDITION CHARACTERISED BY INCREASE OF GLUCOSE LEVEL IN BLOOD (HYPERGLYCAEMIA), HIGH KETONE LEVEL IN BLOOD, AND PRESENCE OF KETONE IN URINE (KETONURIA). • DKA OCCURS WHEN THERE IS INSULIN DEFICIENCY. 1. INSULIN: FUNCTIONS TO LOWERS BLOOD GLUCOSE BY ENHANCE RATE OF GLYCOLYSIS FOR ENERGY PRODUCTION 2. WITHOUT INSULIN: LIVER RAPIDLY BREAKS DOWN FAT TO EMPLOY AS A FUEL SOURCE, RESULTS IN OVERPRODUCTION OF KETONE BODY IN BLOOD AND URINE, LEADING TO DIABETIC KETOACIDOSIS. • MAINLY OCCURS IN DM TYPE 1 BUT NOT UNCOMMON IN DM TYPE 2
- 41. RISK FACTOR OF DKA • PEOPLE WITH DM TYPE 1 SINCE THEIR BODY DOESN’T MAKE INSULINS. • MISSING INSULIN DOSE OFTEN • NOT TAKING INSULIN AS PRESCRIBED • STOMACH ILLNESS • INFECTIONS • HEART DISEASE • RECENT STROKE • BLOOD CLOT IN LUNGS • SERIOUS ILLNESS OR TRAUMA • PREGNANCY • MEDICINES LIKE STEROIDS OR ANTIPSYCHOTIC • USING ILLEGAL DRUGS SUCH AS COCAINE
- 42. SIGNS AND SYMPTOMS OF DKA EARLY SYMPTOMS: • VERY THIRSTY • URINATING A LOT MORE THAN USUAL IF UNTREATED: • FAST, DEEP BREATHING • DRY SKIN AND MOUTH • FLUSHED FACE • FRUITY-SMELLING BREATH • HEADACHE • MUSCLE STIFFNESS OR ACHES • BEING VERY TIRED • NAUSEA AND VOMITING • STOMACH PAIN
- 43. DIAGNOSIS AND TREATMENT OF DKA DIAGNOSIS OF DKA: • BLOOD GLUCOSE LEVEL: >250MG/DL • URINE ANALYSIS: + KETONE, +GLUCOSE • ARTERIAL BLOOD GAS: • BLOOD PH <7.3 • SERUM KETONE: + • SERUM ELECTROLYTE TREATMENT OF DKA • REPLACING FLUID FROM FREQUENT URINATING, HELP DILUTE EXCESS SUGAR IN BLOOD • REPLACING ELECTROLYTES. TOO LITTLE INSULINS CAN LOWER ELECTROLYTE LEVEL • RECEIVING INSULIN. INSULIN REVERSE THE CONDITION THAT CAUSE DKA • TAKING MEDICINES FOR ANY UNDERLYING ILLNESS THAT CAUSE DKA SUCH AS ANTIBIOTIC FOR AN
- 45. CARDIOVASCULAR DISEASE • GENERAL CONDITIONS AFFECTING THE HEART OR BLOOD VESSELS • USUALLY ASSOCIATED WITH BUILD UP OF FATTY DEPOSITS INSIDE ARTERIES (ATHEROSCLEROSIS) AND AN INCREASED RISK OF BLOOD CLOTS • TYPES OF CVD: • CORONARY HEAR DISEASE: BLOCKAGE OF BLOOD VESSELS SUPPLYING THE OXYGEN TO HEART MUSCLE • PERIPHERAL ARTERIAL DISEASE: BLOCKAGE OF BLOOD VESSELS SUPPLYING THE ARMS AND LEGS • RHEUMATIC HEART DISEASE: DAMAGE TO HEART MUSCLE AND HEART VALVES FROM RHEUMATIC FEVER, CAUSED BY STREPTOCOCCAL BACTERIA • CONGENITAL HEART DISEASE: BIRTH DEFECTS THAT AFFECT THE NORMAL DEVELOPMENT AND FUNCTIONING OF HEART.
- 46. RISK FACTOR AND GENERAL SYMPTOMS OF CVD RISK FACTOR • UNHEALTHY DIET • PHYSICAL INACTIVITY • TOBACCO USE • HARMFUL USE OF ALCOHOL • OBESITY GENERAL SYMPTOMS • PAIN OR DISCOMFORT IN CENTRE OF CHEST • PAIN OR DISCOMFORT IN ARMS, LEFT SHOULDER, ELBOWS, JAW OR BACK • HEART ATTACK/STROKE • DIFFICULTY TO BREATH, NAUSEA, FAINTNESS, COLD SWEAT, PALE
- 47. THANK YOU!!