X ray photoelectron spectroscopy
- 1. X-ray photoelectron spectroscopy Electron Spectroscopy for Chemical Analysis (ESCA) Kai M. Siegbahn Obtained Nobel Prize For his work on XPS
- 2. Introduction: X-ray photoelectron spectroscopy (XPS) is a surface- sensitive spectroscopic technique widely used to investigate the chemical composition of surfaces. XPS technique is based on Einstein’s idea about the photoelectric effect, developed around 1905 The concept of photons was used to describe the ejection of electrons from a surface when photons were impinged upon it
- 3. L2,L3 L1 K Incident X-ray Ejected Photoelectron 1s 2s 2p The Photoelectric Process XPS spectral lines are identified by the shell from which electrons were emitted (1s, 2s, 2p etc.) The kinetic energy of the ejected photoelectron KE=hv-BE-F
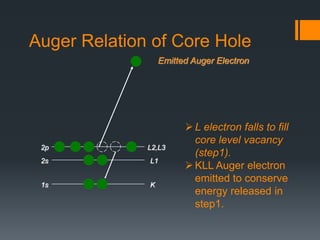
- 4. L2,L3 L1 K Emitted Auger Electron 1s 2s 2p Auger Relation of Core Hole L electron falls to fill core level vacancy (step1). KLL Auger electron emitted to conserve energy released in step1.
- 5. X-Rays Irradiate the sample surface, hitting the core electrons (e-) of the atoms. The X-Rays penetrate the sample to a depth on the order of a micrometer. Useful e- signal is obtained only from a depth of around 10 to 100 Å on the surface. The X-Ray source produces photons with certain energies: MgK photon with an energy of 1253.6 eV AlK photon with an energy of 1486.6 eV Normally, the sample will be radiated with photons of a single energy (MgK or AlK). This is known as a monoenergetic X-Ray beam.
- 6. Why the Core Electrons? An electron near the Fermi level is far from the nucleus, moving in different directions all over the place, and will not carry information about any single atom. Fermi level is the highest energy level occupied by an electron in a neutral solid at absolute 0 temperature. The core e-s are local close to the nucleus and have binding energies characteristic of their particular element. Core e- Valence e- Atom
- 7. How Does XPS Technology Work? A monoenergetic x-ray beam emits photoelectrons from the from the surface of the sample. The x-ray photons The penetration about a micrometer of the sample The XPS spectrum contains information only about the top 10 - 100 Ǻ of the sample. Ultrahigh vacuum environment to eliminate excessive surface contamination. Cylindrical Mirror Analyzer (CMA) measures the KE of emitted e-s. The spectrum plotted by the computer from the analyzer signal. The binding energies can be determined from the peak positions and the elements present in the sample identified.
- 9. COMPONENTS OF XPS: A source of X-rays An ultra high vacuum (UHV) An electron energy analyzer magnetic field shielding An electron detector system A set of stage manipulators
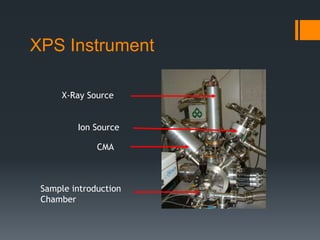
- 10. XPS Instrument X-Ray Source Ion Source CMA Sample introduction Chamber
- 11. WHY WE USE UHV? Remove adsorbed gases from the sample. Eliminate adsorption of contaminants on the sample. Prevent arcing and high voltage breakdown. Increase the mean free path for electrons, ions and photons.
- 12. Dual Anode X-ray Source
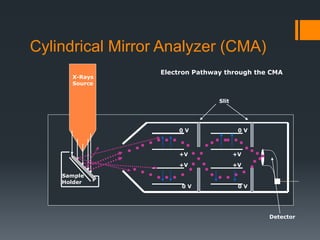
- 13. Cylindrical Mirror Analyzer (CMA) Slit Detector Electron Pathway through the CMA 0 V +V 0 V 0 V 0 V +V +V +V X-Rays Source Sample Holder
- 14. KE versus BE E E E KE can be plotted depending on BE Each peak represents the amount of e-s at a certain energy that is characteristic of some element. 1000 eV 0 eV BE increase from right to left KE increase from left to right Binding energy #ofelectrons (eV)
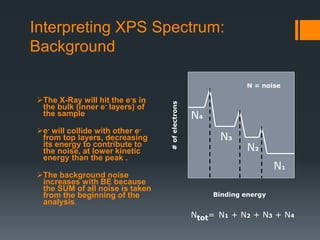
- 15. Interpreting XPS Spectrum: Background The X-Ray will hit the e-s in the bulk (inner e- layers) of the sample e- will collide with other e- from top layers, decreasing its energy to contribute to the noise, at lower kinetic energy than the peak . The background noise increases with BE because the SUM of all noise is taken from the beginning of the analysis. Binding energy #ofelectrons N1 N2 N3 N4 Ntot= N1 + N2 + N3 + N4 N = noise
- 16. XPS peak identification Electronic Effect: Auger lines Chemical shifts X-ray satellites X-ray “Ghost” Energy loss lines
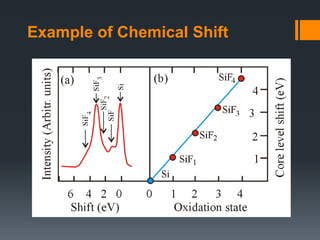
- 17. Example of Chemical Shift
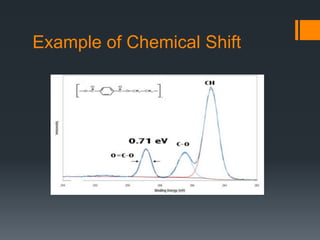
- 18. Example of Chemical Shift
- 19. XPS Study of Changes in the Chemical Composition of Langasite Crystal Thin Surface Layers during Vacuum Annealing The aim of this work was to study the chemical composition of LGS La3Ga5SiO14 langasite crystal wafer surface after thermal vacuum annealing at 650°C and at elevated temperatures (1000°C). To analyze the surface and subsurface layer chemical composition we used X-ray photoelectron spectroscopy (XPS). The 1050°C, 30 min, and 1000 °C, 5 h annealing experiments showed that in both cases the crystals lost color, and the chemical composition of the wafer surface changed: the gallium concentration decreased abruptly
- 20. Figure 1 shows the photoelectron spectra of the crystal surfaces before and after annealing from which the gallium line intensity can be seen to decrease by an order of magnitude.
- 21. Advantages and Disadvantages Advantages Non-destructive technique. Surface Sensitive (10-100 Å). Quantitative measurements are obtained. Provides information about chemical bonding. Elemental mapping. Limitations Very expensive technique. High vacuum is required. Slow processing (1/2 to 8 hours/sample). Large area analysis is required. H and He can not be identified. Data collection is slow 5 to 10 min.
- 22. XPS is used to measure: Elemental composition of the surface (top 1–12 nm usually). Chemical or electronic state of each element in the surface. Uniformity of composition across the top surface (line profiling). Applications in the industry: Failure analysis Polymer surface Corrosion Adhesion Semiconductors Thin film coatings Uses
- 23. References: Siegbahn, K.; Edvarson, K. I. Al (1956). "β-Ray spectroscopy in the precision range of 1 : 1e6". Nuclear Physics Turner, D. W.; Jobory, M. I. Al (1962). "Determination of Ionization Potentials by Photoelectron Energy Measurement". journals.tubitak.gov.tr nanohub.org srdata.nist.gov www.eaglabs.com www.files.chem.vt.edu Bio interface.org www.spectroscopynow.com www.surfaceanalysis.org www.csma.ltd.uk
Editor's Notes
- What information can you obtain from XPS? •Identification of elements near the surface and surface composition •Local chemical environments •Oxidation states of transition metals •Valence band electronic structure •Morphology of thin films XPS detects all elements with (Z) >3. It cannot detect H (Z = 1) or He (Z = 2) because the diameter of these orbitals is so small, reducing the catch probability to almost zero XPS is routinely used to analyze inorganic compounds,metals,semiconductors,polymers, ceramics,etc. Organic chemicals are not routinely analyzed by XPS because they are readily degraded by either the energy of the X-rays or the heat from non-monochromatic X-ray sources
- X-ray photoelectron spectroscopy (XPS) is based on the photoelectric effect. Each atom in the surface has core electron with the characteristic binding energy that is conceptually, not strictly, equal to the ionization energy of that electron. When an Xray beam directs to the sample surface, the energy of the X-ray photon is adsorbed completely by the core electron of an atom. If the photon energy, hn, is large enough, the core electron will then escape from the atom and emit out of the surface. BE= Electron Binding Energy KE= Electron Kinetic Φ= Spectrometer Work Function hn is the X-ray photon energy
- How Does XPS Technology Work? A monoenergetic x-ray beam emits photoelectrons from the from the surface of the sample. The X-Rays either of two energies: Al Ka (1486.6eV) Mg Ka (1253.6 eV) The x-ray photons The penetration about a micrometer of the sample The XPS spectrum contains information only about the top 10 - 100 Ǻ of the sample. Ultrahigh vacuum environment to eliminate excessive surface contamination. Cylindrical Mirror Analyzer (CMA) measures the KE of emitted e-s. The spectrum plotted by the computer from the analyzer signal. The binding energies can be determined from the peak positions and the elements present in the sample identified.
- The sample will be introduced through a chamber that is in contact with the outside environment It will be closed and pumped to low vacuum. After the first chamber is at low vacuum the sample will be introduced into the second chamber in which a UHV environment exists
- The electrons ejected will pass through a device called a CMA. The CMA has two concentric metal cylinders at different voltages. One of the metal cylinders will have a positive voltage and the other will have a 0 voltage. This will create an electric field between the two cylinders. The voltages on the CMA for XPS and Auger e-s are different. When the e-s pass through the metal cylinders, they will collide with one of the cylinders or they will just pass through. If the e-’s velocity is too high it will collide with the outer cylinder If is going too slow then will collide with the inner cylinder. Only the e- with the right velocity will go through the cylinders to reach the detector. With a change in cylinder voltage the acceptable kinetic energy will change and then you can count how many e-s have that KE to reach the detector.
- KE Kinetic Energy (measure in the XPS spectrometer) hv photon energy from the X-Ray source (controlled) Ø spectrometer work function. It is a few eV, it gets more complicated because the materials in the instrument will affect it. Found by calibration. BE is the unknown variable The equation will calculate the energy needed to get an e- out from the surface of the solid. Knowing KE, hv and Ø the BE can be calculated.
- Chemical shift: change in binding energy of a core electron of an element due to a change in the chemical bonding of that element. If a charge q is added to (or removed from) the valence shell due to chemical bond formation, the electrostatic potential felt by the electron inside the atom is changed. • When atom loses valence charge (Si0 --> Si4+ ) BE increases. • When atom gains valence charge (O --> O--) BE decreases. Photoelectron core level peaks in elemental samples occur at the same binding, for example, a gold surface. In compounds, where ionic or covalent bonding occurs, the peak position may shift. For example, Si in pure Si wafer, the binding energy of Si 2p is 99 eV, In contrast, Si in SiO2, the binding energy of Si 2p is 102.3 eV Chemical shift: change in binding energy of a core electron of an element due to a change in the chemical bonding of that element. Core binding energies are determined by electrostatic interaction between it and the nucleus, and change with: • the electrostatic shielding of the nuclear charge from all other electrons in the atom (including valence electrons) • removal or addition of electronic charge as a result of changes in bonding will alter the shielding Atoms of a higher positive oxidation state exhibit a higher binding energy due to the extra coulombic interaction between the photo-emitted electron and the ion core. This ability to discriminate between different oxidation states and chemical environments is one of the major strengths of the XPS technique Binding energy of a core electron depends on number and distribution of valence electrons. •Rule of thumb: higher valence electron density = lower binding energy •Final state effects can revert this trend. •Oxidation leads to higher BE of the cation (e.g. Mn+ in metal oxide) compared to reduced element (less valence electrons). •Typically: +1eV per oxidation state An increase in oxidation state causes the binding energy to increase due to a decrease in the screening of the bound electron from the ion core. The ability of XPS to determine oxidation states is used extensively in catalysis research n principle quantum number l orbital angular quantum number s spin angular quantum number j total angular quantum number (j=l+s)
- Emission from non-monochromatic x-ray sources produces satellite peaks in XPS spectrum at lower BE.