XENOBIOTICS.pptx
- 1. METABOLISM OF XENOBIOTICS Prof (Dr.) Viyatprajna Acharya, MD, PhD Dept. of Biochemistry
- 2. XENOS = FOREIGN XENOBIOTICS = Foreign chemicals Metabolism of xenobiotics = handling of xenobiotics at cellular level Earlier known as detoxification- a misnomer Mostly lipophilic- hence can’t be cleared from body and accumulate.
- 4. Pollutant Substance that increases in quantity due to human activity and adversely affects the environment e.g. CO2, SO2 ,Pb etc
- 5. Contaminant A substance which is not present in nature but released during human activity e.g. DDT, Malathion, plastics
- 6. Xenobiotics that we come across in a single day Toothpaste Phenyl Facewash Soap Deodorant/ anti-perspirant Cosmetics Food- pesticides, dyes, additives Vehicle effluents Industrial pollutants The list is endless…..
- 8. Xenoestrogens 4-Methylbenzylidene camphor (4-MBC) (sunscreen lotions) Butylated hydroxyanisole / BHA (food preservative) Atrazine (weedkiller) Bisphenol A (monomer for polycarbonate plastic and epoxy resin; BPS- insecticides and pesticides Antioxidant in plasticizers Dieldrin (insecticide) DDT (insecticide) Endosulfan (insecticide) Erythrosine / FD&C Red No. 3 Heptachlor (insecticide) • Phenosulfothiazine (a red dye) • Phthalates (plasticizers) • DEHP (plasticizer for PVC) emulsion polymerization; laboratory detergents; pesticides) • Polychlorinated biphenyls / PCBs (in electrical oils, lubricants, adhesives, paints) • Parabens (lotions) • Lindane / hexachlorocyclohexane (insecticide) • Methoxychlor (insecticide) • Nonylphenol and derivatives (industrial surfactants; emulsifiers for
- 10. Statistically significant against uncontaminated water Samples of scratched pet bottles, baby feeding bottle was ~1000 times; BPA in climate exposed water was maximum Hot water poured in polythene bag was worst- 1 crore times!!!
- 12. Metabolism Aim is to make xenobiotic inactive or less harmful Make them hydrophilic- easy excretion from body 2 phases Site-liver
- 13. PHASE-I REACTION Hydroxylation- MC type; catalysed by Cytochrome P450 enzymes (monooxygenases) Other reactions- Hydrolysis, oxidation, reduction, deamination, Peroxidation, epi-oxidation
- 14. Oxidation reactions Alcohol metabolism- ADH and ALDH Oxidation may produce more toxic materials e.g. Methanol→ Formic acid Ethylene glycol → Oxalic acid
- 15. Reduction reactions Nitro compounds are reduced and detoxified mostly They are reduced to their amines Aldehydes and ketones are reduced to alcohols Nitrobenzene → Aniline Picric acid → Picramic acid Para-nitrophenol → p-aminophenol
- 16. Hydrolysis Esters, amines, hydrazines, amides, glycosidic bonds and carbamates Aspirin, acetanilide, procaine, xylocaine, aliphatic esters, DFP
- 17. Phase-II reactions Further modification and conjugation-makes the substance more water soluble and helps in excretion Mostly contain hydroxyl group (-OH), amino (-NH2) and carboxyl groups (-COOH) Acetylation Methylation Sulfation Conjugation with Glycine Conjugation with glucuronic acid
- 18. Glucuronidation UDP glucuronic acid Bilirubin- in presence of glucuronyl transferase BMG and BDG are produced Other products undergoing glucuronidation- i. Acetylaminofluorene (carcinogen), ii. Aniline (dye) iii. Benzoic acid iv. Phenol v. Meprobamate
- 19. Sulphation PAPS- 3-phospho-adenosine-5-phosphosulphate/ active Sulphur is the sulphate donor Alcohols, arylamines, phenols are sulphonated
- 20. Conjugation with Glutathione Glutathione-s-transferase enzyme catalyzes Carcinogens are metabolised ↓GSH- increased tissue damage
- 21. Acetylation Acetyl CoA is the acetyl group donor Acetyltransferase enzyme INH is acetylated before excretion Polymorphic forms- Slow and fast acetylators Slow acetylators – more toxic effects of INH
- 22. Methylation S-adenosyl-methionine (SAM)- methyl group donor Enzyme –methyltransferase
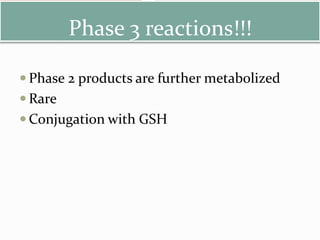
- 23. Phase 3 reactions!!! Phase 2 products are further metabolized Rare Conjugation with GSH
- 24. Cytochrome P 450/ Monooxygenases Heme protein In reduced state bind with CO and absorb light maximally at 450nm Versatile enzyme- catalyze a wide range of products like drugs, carcinogens, pesticides etc. and endogenous metabolites- FA, eicosanoids, steroids etc
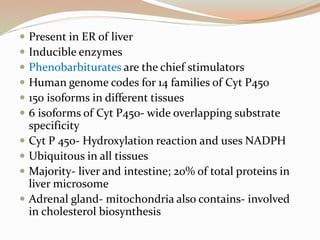
- 25. Present in ER of liver Inducible enzymes Phenobarbiturates are the chief stimulators Human genome codes for 14 families of Cyt P450 150 isoforms in different tissues 6 isoforms of Cyt P450- wide overlapping substrate specificity Cyt P 450- Hydroxylation reaction and uses NADPH Ubiquitous in all tissues Majority- liver and intestine; 20% of total proteins in liver microsome Adrenal gland- mitochondria also contains- involved in cholesterol biosynthesis
- 26. Mechanism of induction of Cyt-P450 mRNA transcription mRNA stabilization Enzyme stabilization
- 27. Nomenclature
- 28. Some isoforms show genetic polymorphism CYP2A6- metabolises nicotine Some allelic forms are poor metabolizers People having this have always high nicotine content in their blood- hence saved from nicotine addiction
- 29. Factors affecting metabolism of xenobiotics Species difference Enzyme activities may vary Age and gender Genetic make-up Inducers & inhibitors
- 30. XENOBIOTICS TOXICITY Reactive metabolites Mutation-carcinogenesis Xenobiotics may themselves become carcinogens Act as hapten-immunogenicity Epoxide derivatives- dihydrodiols- highly reactive and mutagenic
- 31. For more PPT on Medical Biochemistry- www.drvpacharya.com
- 32. For more PPT on Medical Biochemistry www.drvpacharya.com
Editor's Notes
- India is at higher risk due to heavy sunlight exposure (7KWH on an avg) where plastic water and food containers are freely transported. Water poured in polythene simulated hot food being carried in polythenes. Need to give a thought regarding cold drink bottles too.
- The stability of a given mRNA transcript is determined by the presence of sequences within an mRNA known as cis-elements, which can be bound by trans-acting RNA-binding proteins to inhibit or enhance mRNA decay.