ENERGY AS HEAT
- 1. Energy as Heat • A sample can transfer energy to another sample. • One of the simplest ways energy is transferred is as heat. • Heat is the energy transferred between objects that are at different temperatures • Though energy has many different forms, all energy is measured in units called joules (J).
- 2. • Temperature is a measure of how hot (or cold) something is; specifically, a measure of the average kinetic energy of the particles in an object. • When samples of different temperatures are in contact, energy is transferred from the sample that has the higher temperature to the sample that has the lower temperature.
- 3. • Temperature is an intensive property, which means that the temperature of a sample does not depend on the amount of the sample. • Heat is an extensive property, which means that the amount of energy transferred as heat by a sample depends on the amount of the sample.
- 4. • Changes in energy content can be determined. • If 73 J of energy enter a piece of silver and no change in pressure occurs, we know that the enthalpy of the silver has increased by 73 J. • Enthalpy, which is represented by the symbol H, is the total energy content of a sample.
- 5. • Enthalpy is the sum of the internal energy of a system plus the product of the system’s volume multiplied by the pressure that the system exerts on its surroundings.
- 6. • The particles in a sample are in constant motion. • The enthalpy of a sample includes the total kinetic energy of its particles. • Both the total and average kinetic energies of a substance’s particles are important to chemistry, because these quantities account for every particle’s kinetic energy.
- 7. • Molar heat capacity can be used to determine the enthalpy change of a sample. • The molar heat capacity of a pure substance is the energy as heat needed to increase the temperature of 1 mol of the substance by 1 K. • Molar heat capacity has the symbol C and the unit J/K•mol.
- 8. • When a substance receives energy in the form of heat, its enthalpy increases and the kinetic energy of the particles that make up the substance increases. • The direction in which any particle moves is not related to the direction in which its neighboring particles move. The motions of these particles are random.
- 9. • Because enthalpy is the total energy of a system, it is an important quantity. • The only way to measure energy is through a change. Absolute values cannot be measured. • The enthalpy change for one mole of a pure substance is called molar enthalpy change.
- 10. • When a pure substance is only heated or cooled, the amount of heat involved is the same as the enthalpy change. ∀∆H = q for the heating or cooling of substances
- 11. • A change in enthalpy during a reaction depends on many variables. • Temperature is one of the most important variables. • To standardize the enthalpies of reactions, data are often presented for reactions in which both reactants and products have the standard thermodynamic temperature of 25.00°C or 298.15 K.
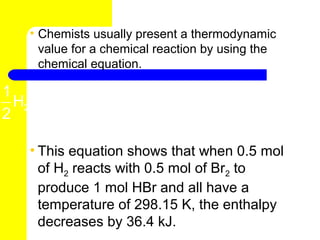
- 12. • Chemists usually present a thermodynamic value for a chemical reaction by using the chemical equation. 1 1 H2 ( g ) + Br2 ( l ) → HBr ( g ) ∆H = -36.4 kJ 2 2 • This equation shows that when 0.5 mol of H2 reacts with 0.5 mol of Br2 to produce 1 mol HBr and all have a temperature of 298.15 K, the enthalpy decreases by 36.4 kJ.
- 13. • For the H2 and Br2 reaction, in which ∆H is negative, the total energy of the reaction decreases. • The energy is released as heat by the system. • If the reaction was endothermic, energy in the form of heat would be absorbed by the system and the enthalpy would increase.
- 14. • The experimental measurement of an enthalpy change for a reaction is called calorimetry. • Calorimetry is the measurement of heat-related constants, such as specific heat or latent heat • Combustion reactions are always exothermic. • The enthalpy changes of combustion reactions are determined using a bomb calorimeter. • A calorimeter Is a device used to measure the heat absorbed or released in a chemical or physical change
- 16. • Inside the pressurized oxygen atmosphere of a bomb calorimeter, most organic matter, including food, fabrics, and plastics, will ignite easily and burn rapidly. • Sample sizes are chosen so that there is excess oxygen during the combustion reactions. • Under these conditions, the reactions go to completion and produce carbon dioxide, water, and possibly other compounds.
- 17. • Any two processes that both start with the same reactants in the same state and finish with the same products in the same state will have the same enthalpy change. • Hess’s law states that the overall enthalpy change in a reaction is equal to the sum of the enthalpy changes for the individual steps in the process.
- 18. • When phosphorus is burned in excess chlorine 4 mol of phosphorus pentachloride, PCl5, is synthesized. P4(s) + 10Cl2(g) → 4PCl5(g) ∆H = -1596 kJ • Phosphorus pentachloride may also be prepared in a two-step process. Step 1: P4(s) + 6Cl2(g) → 4PCl3(g) ∆H = -1224 kJ Step 2: PCl3(g) + Cl2(g) → PCl5(g) ∆H = -93 kJ
- 19. • The second reaction must take place four times for each occurrence of the first reaction in the two-step process. • This two-step process is more accurately described by the following equations. P4(s) + 6Cl2(g) → 4PCl3(g) ∆H = -1224 kJ 4PCl3(g) + 4Cl2(g) → 4PCl5(g) ∆H = 4(-93 kJ) = -372 kJ
- 20. • So, the total change in enthalpy by the two-step process is as follows: (-1224 kJ) + (-372 kJ) = -1596 kJ • This enthalpy change, ∆H, for the two-step process is the same as the enthalpy change for the direct route of the formation of PCl5. • This example is in agreement with Hess’s law.
- 21. • The enthalpy of the formation of CO, when CO2 and solid carbon are reactants, is found using the equations below. 2C(s) + O2(g) → 2CO(g) ∆H = -221 kJ C(s) + O2(g) → CO2(g) ∆H = -393 kJ • You cannot simply add these equations because CO2 would not be a reactant.
- 22. • Adding the two equations gives the equation for the formation of CO by using CO2 and C. 2C(s) + O2(g) → 2CO(g) ∆H = – 221 kJ CO2(g) → C(s) + O2(g) ∆H = 393 kJ 2C(s) + O2(g) + CO2(g) → 2CO(g) + C(s) + O2(g) ∆H = 172 kJ • Oxygen and carbon that appear on both sides of the equation can be canceled.
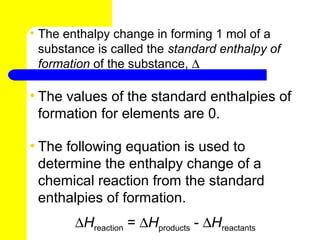
- 23. • The enthalpy change in forming 1 mol of a substance is called the standard enthalpy of formation of the substance, ∆ Hf0 • The values of the standard enthalpies of formation for elements are 0. • The following equation is used to determine the enthalpy change of a chemical reaction from the standard enthalpies of formation. ∆Hreaction = ∆Hproducts - ∆Hreactants