Modul 2
Modul 2
Uploaded by
Aditya Whisnu HeryudhantoCopyright:
Available Formats
Modul 2
Modul 2
Uploaded by
Aditya Whisnu HeryudhantoCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Copyright:
Available Formats
Modul 2
Modul 2
Uploaded by
Aditya Whisnu HeryudhantoCopyright:
Available Formats
Chapter 7 Reaction Mechanisms, Pathways, Bioreactions, and Bioreactors
Problem 7-11B Beef catalase has been used to accelerate the decomposition of hydrogen
peroxide to yield water and oxygen [Chem. Eng. Educ., 5, 141 (1971)]. The concentration of
hydrogen peroxide is given as a function of time for a reaction mixture with a pH of 6.76
maintained at 30C.
t (min)
C H2O2 (mol/L)
0
0.02
10
0.01775
20
0.0158
50
0.0106
100
0.005
(a) Determine the Michaetis-Menten parameters Vmax and KM.
(b) If the total enzyme cancentration is tripled, what will the substrate concentration be after
20 minutes?
(c) How could you make this problem more difficult?
(d) List ways you can work this problem incorrectly.
Problem 7-12B It has been observed that substrate inhibition occurs in the following
enzymatic reaction:
1
+
2
+
3
+
4
. +
5
. +
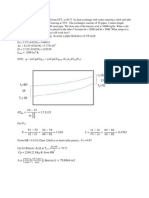
(a) Show that the rate law for the sequence above is consistent with the plot in Figure P7-12
of -rs, (mmol/L.min) versus the substrate concentration S (mmol/L).
Figure P7-12. Michealis-Menten plot for substrate inhibition
Problem 7-16B The production of a product P from a particular gram negative bacteria
follows the Monod growth law
+
= 0.5 g/g.
=
with max = 1 h-1 KM = 0.25 g/dm3, and Yc/s
(a). The reaction is to be carried out in a batch reactor with the initial cell concentralion of Cc0
= 0.1 g/dm3 and substrate concentration of Cs0 = 20 g/dm3.
Cc = Cc0 + Yc/s (Cs0 Cs)
Plot rs, -rc, Cs and Cc as a function of time.
(b) Redo part (a) and use a logistic growth law.
)
and plot Cc and rc as a function of time. The term , is the maximum cell mass and is called
the carrying capacity and is equal to C = 1.0 g/dm3. Can you find an analytical solution for
the batch reactor? Compare with part (a) for C = Yc/s Cs0 + Cc0.
= (1
(c) The reaction is now to be carried out in a CSTR with Cs0 = 20 g/dm3 and Cc0 = 0. What is
the dilution rate at which washout occurs?
(d) For the conditions in part (c), what is the dilution rate that will give the maximum product
rate (g/h) if Yp/c = 0.15 g/g? What are the concentrations Cc, Cs, Cp, and rs at this value of D?
(e) How would your answers to (c) and (d) change if cell death could not be neglected with kd
= 0.02 h-1?
(f) How would your answers to (c) and (d) change if maintenance could not be neglected with
m = 0.2 g/h/dm3?
(g) List ways you can work this problem incorrectly.
(h) How could you make this problem more difficult?
Problem 7-23A A CSTR is being operated at steady state. The cell growth follows the Monod
growth law without inhibition. The exiting substrate and cell concentrations are measured as a
function of the volumetric flow rate (represented as the dilution rate), and the results are shown
below. Of course, measurements are not taken until steady state is achieved after each change
in the flow rate. Neglect substrate consumption for maintenance and the death rate, and assume
that Yp/c is zero. For run 4, the entering substrate concentration was 50 g/dm3 and the volumetric
flow rate of the substrate was 2 dm3/s.
Run
Cs (g/dm3)
D (day-1)
Cc (g/dm3)
1
1
1
0.9
2
3
1.5
0.7
3
4
1.6
0.6
4
10
1.8
4
(a) Determine the Monod growth parameters max and Ks.
(b) Estimate the stoichiometric coeficients, Yc/s and Ys/c.
(c) List ways you can work this problem incorrectly.
(d) How could you make this problem more difficult?
You might also like
- 2 - 5 - Catalysis Catalytic ReactorsDocument34 pages2 - 5 - Catalysis Catalytic Reactorsk.shilpya30No ratings yet
- Bose InvoiceDocument2 pagesBose Invoicef2000240No ratings yet
- BT HPTDocument31 pagesBT HPTLinh NguyenNo ratings yet
- Diffusion Effects in Enzymes Immobilized in A Porous MatrixDocument18 pagesDiffusion Effects in Enzymes Immobilized in A Porous MatrixAegee0% (1)
- ChE132 Case Study WriteupDocument20 pagesChE132 Case Study WriteuphuyNo ratings yet
- CIV541 Assignment - 1 General As ProblemsDocument3 pagesCIV541 Assignment - 1 General As ProblemsBalsher Sidhu0% (1)
- 4501 Homework04solDocument9 pages4501 Homework04solDaudi Erasto MlangiNo ratings yet
- Cellic CTec3 HS Application Sheet NADocument5 pagesCellic CTec3 HS Application Sheet NADaniel RivaldiNo ratings yet
- Heat Transfer ProblemDocument2 pagesHeat Transfer ProblemRenz Roger Esteves BuendichoNo ratings yet
- Fogler Ejemplo 8-7Document7 pagesFogler Ejemplo 8-7Niels Estrada VilaNo ratings yet
- Workshop Leaching and WashingDocument2 pagesWorkshop Leaching and WashingAnyela CiroNo ratings yet
- CHE3044F: Reactor Design 1: TUTORIAL 7, 2012: April 29, 2013Document2 pagesCHE3044F: Reactor Design 1: TUTORIAL 7, 2012: April 29, 2013nmhatityeNo ratings yet
- GATE HELPLINE Bioprocess Engineering MCQ IDocument4 pagesGATE HELPLINE Bioprocess Engineering MCQ ISanthosh Kalash100% (9)
- Hammer Mill Vertica PDFDocument6 pagesHammer Mill Vertica PDFAditya Whisnu HeryudhantoNo ratings yet
- SQL Questions & AnswersDocument9 pagesSQL Questions & AnswersTsehayou SieleyNo ratings yet
- Problem Set 1Document8 pagesProblem Set 1Bj LarracasNo ratings yet
- Tutorial # 1 - KineticsDocument7 pagesTutorial # 1 - KineticsbebsybiswezNo ratings yet
- Lecture 3Document21 pagesLecture 3alyssaNo ratings yet
- CHEM3002 Tutorial Sheet 3Document1 pageCHEM3002 Tutorial Sheet 3Sunmoon Al-HaddabiNo ratings yet
- A Novel Reverse Flow Strategy For Ethylbenzene Dehydrogenation in A Packed-Bed ReactorDocument17 pagesA Novel Reverse Flow Strategy For Ethylbenzene Dehydrogenation in A Packed-Bed ReactorMuhammad Akbar FahleviNo ratings yet
- Felix Termodinamica Quimica ch03Document104 pagesFelix Termodinamica Quimica ch03Amilcar Pereira CardosoNo ratings yet
- 8.4 Continuous Reactors: 8.4.1 Steady-State Chemostat (CHEMOSTA)Document33 pages8.4 Continuous Reactors: 8.4.1 Steady-State Chemostat (CHEMOSTA)Hana HamidNo ratings yet
- Soal Nomor 18Document2 pagesSoal Nomor 18Mawaddah Nur TambakNo ratings yet
- Sample Problem #5Document10 pagesSample Problem #5DozdiNo ratings yet
- Given: Stagnant Vapor Film of 0.1-Inch (0.00833-ft) Thickness, Containing 30 Mol% Toluene andDocument2 pagesGiven: Stagnant Vapor Film of 0.1-Inch (0.00833-ft) Thickness, Containing 30 Mol% Toluene andMark Lester RealNo ratings yet
- CH 14Document82 pagesCH 14Sadie Hnatow75% (4)
- Tugas Pap Kel3Document9 pagesTugas Pap Kel316-125 Ruth Ria RistaNo ratings yet
- B11Document5 pagesB11Amanda GrazieleNo ratings yet
- Bio-Chemical Engineering Thermodynamics 552 Answer Page PDFDocument550 pagesBio-Chemical Engineering Thermodynamics 552 Answer Page PDFNaveenNo ratings yet
- Taller Parcial de ReacccionesDocument7 pagesTaller Parcial de ReacccionesAndresFelipeSotoNo ratings yet
- Assignment Answer Scheme PDFDocument17 pagesAssignment Answer Scheme PDFHizami Mohammad Noor100% (2)
- Gas Absorption PDFDocument93 pagesGas Absorption PDFIngeniería Industrias Alimentarias Itsm100% (1)
- The BET IsothermDocument7 pagesThe BET IsothermBasemNo ratings yet
- Cooking PotatoDocument12 pagesCooking Potatonovi_wijaya_2No ratings yet
- KineticsDocument123 pagesKineticssamueloNo ratings yet
- Assignment 1Document2 pagesAssignment 1boiroyNo ratings yet
- Cre-Ii Lectppts Partii CatreactionsDocument325 pagesCre-Ii Lectppts Partii CatreactionsdhruvNo ratings yet
- Sample Problems On Ideal Reactor ModelsDocument7 pagesSample Problems On Ideal Reactor ModelsGirllietopsyNo ratings yet
- Chapter 1aDocument8 pagesChapter 1aJan Angela Almiranes0% (1)
- Example 2: Over What Range of Conversions Are The Plug-Flow Reactor and CSTR Volumes Identical?Document8 pagesExample 2: Over What Range of Conversions Are The Plug-Flow Reactor and CSTR Volumes Identical?Alejandra SanchezNo ratings yet
- Stirred Tank HeaterDocument34 pagesStirred Tank HeaterIman Haerudin100% (1)
- Problem 5.30 Levenspiel - David FS - 2315201015Document5 pagesProblem 5.30 Levenspiel - David FS - 2315201015Arief WidjajaNo ratings yet
- Tutorial 4.2 (Transfer Function)Document3 pagesTutorial 4.2 (Transfer Function)Griezmann HaziqNo ratings yet
- Phychem 1 Review 1 Sept 2015Document2 pagesPhychem 1 Review 1 Sept 2015Jupert Jasser AbellanaNo ratings yet
- 2.10 A Substrate Is Decomposed in The Presence of An Enzyme According To The Michaelis-MentenDocument2 pages2.10 A Substrate Is Decomposed in The Presence of An Enzyme According To The Michaelis-MentenEureca ParraNo ratings yet
- Chapter 3 - Industrial Hygiene - FinalDocument41 pagesChapter 3 - Industrial Hygiene - FinalSatvik SaxenaNo ratings yet
- R09 Set No. 2Document8 pagesR09 Set No. 2Shakoor MalikNo ratings yet
- TUTORIAL Mass Transfer PrincipleDocument7 pagesTUTORIAL Mass Transfer PrincipleXin-YiWoon0% (1)
- Thermodynamics TutorialDocument2 pagesThermodynamics TutorialMuhamad Hazim Zaaba0% (1)
- Lab 5 Production of Ethyl ChlorideDocument19 pagesLab 5 Production of Ethyl ChloridelynNo ratings yet
- Assignment 1 MT1 2016Document13 pagesAssignment 1 MT1 2016Ushnish Rana100% (1)
- Нome Assignment 4Document3 pagesНome Assignment 4Abdu M. HabsyiNo ratings yet
- Exercise Chapter6Document23 pagesExercise Chapter6Izzat Rawaida0% (1)
- Fundamentals of Heat and Mass Transfer 6th Edition-901-1000-51-100Document50 pagesFundamentals of Heat and Mass Transfer 6th Edition-901-1000-51-100abibas olaNo ratings yet
- Tutorial 3 - CrystallizationDocument3 pagesTutorial 3 - CrystallizationAhmad Muzammil25% (4)
- Humidification and Air Conditioning: Lecture No. 8Document6 pagesHumidification and Air Conditioning: Lecture No. 8Anonymous UFa1z9XUANo ratings yet
- CH 6701 Cre IiDocument230 pagesCH 6701 Cre IiVaibhav Gupta100% (1)
- TUTORIAL-on Absorption - 2018 - SolutionDocument2 pagesTUTORIAL-on Absorption - 2018 - SolutionMayank Prasad100% (1)
- Chemical Reaction Kinetics: Concepts, Methods and Case StudiesFrom EverandChemical Reaction Kinetics: Concepts, Methods and Case StudiesNo ratings yet
- GATE - 2003 Chemical Engineering Useful DataDocument12 pagesGATE - 2003 Chemical Engineering Useful DataCharishmaNo ratings yet
- Nama: Nuri Irianti NIM: 1009035009 Prodi: Teknik LingkunganDocument3 pagesNama: Nuri Irianti NIM: 1009035009 Prodi: Teknik LingkunganNuri IriantiNo ratings yet
- Homework 03 - Sem 1 - 2020-2021Document8 pagesHomework 03 - Sem 1 - 2020-2021Kim HânNo ratings yet
- AE 98 Civ B5 Water Supply and Wastewater Treatment May 2002Document13 pagesAE 98 Civ B5 Water Supply and Wastewater Treatment May 2002mkaswaNo ratings yet
- Variasi SilikaDocument1 pageVariasi SilikaAditya Whisnu HeryudhantoNo ratings yet
- Residue Avoidance Program: Feed Mixing SystemsDocument4 pagesResidue Avoidance Program: Feed Mixing SystemsAditya Whisnu HeryudhantoNo ratings yet
- PR Termo Indah 3.25 Dan 3.32Document8 pagesPR Termo Indah 3.25 Dan 3.32Aditya Whisnu HeryudhantoNo ratings yet
- PR PPK No.2&Amp 7Document3 pagesPR PPK No.2&Amp 7Aditya Whisnu HeryudhantoNo ratings yet
- Intro ME927Document5 pagesIntro ME927Aditya Whisnu HeryudhantoNo ratings yet
- FB Hammermill en DataDocument8 pagesFB Hammermill en DataAditya Whisnu Heryudhanto100% (1)
- List of International Competitions 2017 (Rule 35.9)Document4 pagesList of International Competitions 2017 (Rule 35.9)Aditya Whisnu HeryudhantoNo ratings yet
- 05 Mikro Alga PDFDocument9 pages05 Mikro Alga PDFMaelstromNo ratings yet
- XMTRP 1703010Document5 pagesXMTRP 1703010Aditya Whisnu HeryudhantoNo ratings yet
- Cambridge International AS & A Level: Biology October/November 2023 2 HoursDocument24 pagesCambridge International AS & A Level: Biology October/November 2023 2 HoursZhemaiah OzeNo ratings yet
- MHRD With Slide EffectsDocument34 pagesMHRD With Slide EffectsNatarajan NalanthNo ratings yet
- Fast Track Pro Drivers Read MeDocument3 pagesFast Track Pro Drivers Read MeMatt JerniganNo ratings yet
- Women Entrepreneurs - A Mirage of Indian WomenDocument1 pageWomen Entrepreneurs - A Mirage of Indian WomenRajni GargNo ratings yet
- FishingDocument23 pagesFishingsingaravelNo ratings yet
- Evolution of RegionDocument2 pagesEvolution of RegiondasnavneepNo ratings yet
- Hydraulic Crane Bumpers IntroductionDocument2 pagesHydraulic Crane Bumpers IntroductionManu GimenezNo ratings yet
- 2021 HRMT Essay TurnDocument4 pages2021 HRMT Essay TurngillNo ratings yet
- PDF Sample Problem1Document34 pagesPDF Sample Problem1ERVIN JAMES ABULOCNo ratings yet
- Third Cycle ProgramsDocument3 pagesThird Cycle Programsbeauty rajaNo ratings yet
- Condition of The Surface of Commutators and Rings-Roughness: Technical Note Sta Be 16-1 GBDocument2 pagesCondition of The Surface of Commutators and Rings-Roughness: Technical Note Sta Be 16-1 GBloulou_beNo ratings yet
- Assignment 02 - Basics of PythonDocument5 pagesAssignment 02 - Basics of Pythonhina InamNo ratings yet
- Japan Auctions GradesDocument5 pagesJapan Auctions GradesAlzieNo ratings yet
- Fact Sheet - Electrician (General)Document4 pagesFact Sheet - Electrician (General)Saravanan Rasaya100% (1)
- Kitchen Aid Kebs208 Oven PartsDocument7 pagesKitchen Aid Kebs208 Oven PartsfiskitNo ratings yet
- HDD Hitachi HTS547564A9E384Document170 pagesHDD Hitachi HTS547564A9E384scrib3dsNo ratings yet
- Marine ONYX® Systems: Approved Components For Marine ApplicationsDocument4 pagesMarine ONYX® Systems: Approved Components For Marine ApplicationsDANIEL HERNANDEZ HUASASQUICHENo ratings yet
- Psych Rome TryDocument13 pagesPsych Rome TryAmira BagumbaranNo ratings yet
- Data Collection MethodsDocument62 pagesData Collection MethodsShahid ShaShaNo ratings yet
- How Does Grit Impact College Students Academic Achievement in ScienceDocument11 pagesHow Does Grit Impact College Students Academic Achievement in SciencePATRICIA KATE CELSONo ratings yet
- 1000 Ways To Pack The Bin PDFDocument50 pages1000 Ways To Pack The Bin PDFafsarNo ratings yet
- Ford Strategic Mangement - Chevy VoltDocument2 pagesFord Strategic Mangement - Chevy VoltVikram Santhanam100% (5)
- Chapter 4 OrganizingDocument39 pagesChapter 4 OrganizingHanzel Dy NietesNo ratings yet
- ScriptDocument3 pagesScriptRhyzzhy Swythy PhsyclypyrNo ratings yet
- Ipaml FinalsDocument3 pagesIpaml FinalsQuenneeNo ratings yet
- Politics of Globalisation QP 2019 - TutorialsDuniyaDocument5 pagesPolitics of Globalisation QP 2019 - TutorialsDuniyasatyam kumarNo ratings yet
- D71862GC10 1001 UsDocument4 pagesD71862GC10 1001 UsbugzbinnyNo ratings yet
- Colector Analizador de Vibraciones Falcon PDF 2 MBDocument6 pagesColector Analizador de Vibraciones Falcon PDF 2 MBGabriel Alexis MartínezNo ratings yet