0 ratings0% found this document useful (0 votes)
54 views112.1 Exercise 9
112.1 Exercise 9
Uploaded by
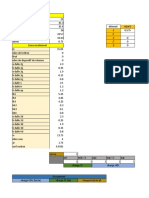
Julie Ann Felices1) Five trials of titrating KIO4 solutions with standardized Na2S2O3 were conducted under various conditions of temperature and solvent.
2) The results, including final and initial buret readings and volumes used, were recorded to calculate the molarity and average molarity of the KIO4 solutions.
3) Additional experiments were performed to determine the solubility product (KSP) values, activity coefficients (γ), and ionic strengths (σ) of KIO4 in different solvents and temperatures.
Copyright:
© All Rights Reserved
Available Formats
Download as XLSX, PDF, TXT or read online from Scribd
112.1 Exercise 9
112.1 Exercise 9
Uploaded by
Julie Ann Felices0 ratings0% found this document useful (0 votes)
54 views3 pages1) Five trials of titrating KIO4 solutions with standardized Na2S2O3 were conducted under various conditions of temperature and solvent.
2) The results, including final and initial buret readings and volumes used, were recorded to calculate the molarity and average molarity of the KIO4 solutions.
3) Additional experiments were performed to determine the solubility product (KSP) values, activity coefficients (γ), and ionic strengths (σ) of KIO4 in different solvents and temperatures.
Original Description:
Data excel
Original Title
112.1-Exercise-9
Copyright
© © All Rights Reserved
Available Formats
XLSX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
1) Five trials of titrating KIO4 solutions with standardized Na2S2O3 were conducted under various conditions of temperature and solvent.
2) The results, including final and initial buret readings and volumes used, were recorded to calculate the molarity and average molarity of the KIO4 solutions.
3) Additional experiments were performed to determine the solubility product (KSP) values, activity coefficients (γ), and ionic strengths (σ) of KIO4 in different solvents and temperatures.
Copyright:
© All Rights Reserved
Available Formats
Download as XLSX, PDF, TXT or read online from Scribd
Download as xlsx, pdf, or txt
0 ratings0% found this document useful (0 votes)
54 views3 pages112.1 Exercise 9
112.1 Exercise 9
Uploaded by
Julie Ann Felices1) Five trials of titrating KIO4 solutions with standardized Na2S2O3 were conducted under various conditions of temperature and solvent.
2) The results, including final and initial buret readings and volumes used, were recorded to calculate the molarity and average molarity of the KIO4 solutions.
3) Additional experiments were performed to determine the solubility product (KSP) values, activity coefficients (γ), and ionic strengths (σ) of KIO4 in different solvents and temperatures.
Copyright:
© All Rights Reserved
Available Formats
Download as XLSX, PDF, TXT or read online from Scribd
Download as xlsx, pdf, or txt
You are on page 1of 3
Standardization Titration of KIO4 solutions with standardized Na2S2O3
Trial Temperature (C)
Solution Solvent
I II I
Final buret reading, mL 55 61 1 distilled H2O 19
Initial buret reading, mL 50 55 2 0.20 M NaNO3 31
Volume used, mL 5 6 3 0.10 M NaNO3 31
Mass 0.0645 0.0662 4 0.20 M KNO3 19
Molarity 0.46627 0.3988 5 0.10 M KNO3 19
average molarity 0.43253
Activity
Solvent
IO4-
distilled H2O 0.0335210843
0.20 M NaNO3 0.0435233434
0.10 M NaNO3 0.0451453313
0.20 M KNO3 0.0110835843
0.10 M KNO3 0.0170308735
Coated with counterion - salting in
Common ion - lower solubility than distilled water
ardized Na2S2O3 Volume of Na2S2O3, mL
Temperature (C) Volume of aliquot, mL Vfinal
II I II I II
19 10 10 89.2 95.4
31 10 10 70.1 57.2
31 10 10 79.4 66
19 10 10 54 56.1
19 10 10 82.8 59
KSP I gamma sigma
K+
0.0335210843 0.0011236631 0.6024023807 0.4026630347 0.055645670
0.0435233434 0.0018942814 0.8024023807 0.3499882785 0.0542412939
0.0451453313 0.002038 0.7024023807 0.3744638539 0.0642727482
0.2110835843 0.0023395627 0.8024023807 0.3499882785 0.0705864962
0.1170308735 0.001993138 0.7024023807 0.3744638539 0.0792827946
Vinitial Vol used Average
I II I II
82.8 89.4 6.4 6 6.2
61.2 50 8.9 7.2 8.05
70.1 58.6 9.3 7.4 8.35
52 54 2 2.1 2.05
79.4 56.1 3.4 2.9 3.15
You might also like
- Lec 4-1 Well Log CorrelationDocument60 pagesLec 4-1 Well Log CorrelationMangar Mawut100% (2)
- Specifications: Latham Expansion Joint Cover Suggested Architectural SpecificationsDocument5 pagesSpecifications: Latham Expansion Joint Cover Suggested Architectural SpecificationsDavid Alexander Calderón ArreguiNo ratings yet
- Bahan Katoda Subhan NMCDocument9 pagesBahan Katoda Subhan NMCachmad subhanNo ratings yet
- Chem 112.1 - Exer 9 Table and AnswersDocument7 pagesChem 112.1 - Exer 9 Table and AnswersGerry Mark GubantesNo ratings yet
- Tugas - 2: Surface FacilitiesDocument5 pagesTugas - 2: Surface FacilitiesStephanie VirganaNo ratings yet
- Appendixs ADocument188 pagesAppendixs ABurhanudin MuizNo ratings yet
- Hidro - Kel 6 MektanDocument19 pagesHidro - Kel 6 MektanAjeng PramestiNo ratings yet
- Curva Ton-Ley Angelica GlobalDocument6 pagesCurva Ton-Ley Angelica GlobalOliver Ventocilla PadillaNo ratings yet
- Adsorción IBQDocument13 pagesAdsorción IBQkarimefosterNo ratings yet
- Book 1Document3 pagesBook 1Lilik UtamaNo ratings yet
- Titrasi Asam Lemah Dan Basa Kuat IsianDocument8 pagesTitrasi Asam Lemah Dan Basa Kuat IsianFara Rizky Ananda PutriNo ratings yet
- Systimization and Circuitization Creation Case StudyDocument12 pagesSystimization and Circuitization Creation Case Studyahmed sobhyNo ratings yet
- Acid - HCL CalculationDocument9 pagesAcid - HCL CalculationDidin DelgadoNo ratings yet
- University of KarachiDocument5 pagesUniversity of Karachitaha zafarNo ratings yet
- BoilerDocument29 pagesBoilerhonchoabhiNo ratings yet
- Mineral Water Calculator v5Document7 pagesMineral Water Calculator v5mauricio0327No ratings yet
- Perhitungan Difusivitas IntegralDocument12 pagesPerhitungan Difusivitas IntegralamosagungNo ratings yet
- Material Version MATTDocument22 pagesMaterial Version MATTSuphawit KeaseNo ratings yet
- Anorthite Hydrolysis: Mike Borr Geol 428 Geochemistry NDSU Fall 2012Document25 pagesAnorthite Hydrolysis: Mike Borr Geol 428 Geochemistry NDSU Fall 2012yuri huamanguillas saenzNo ratings yet
- Date Table: Universidad Nacional de Ancash "Santiago Antunez de Mayolo"Document1 pageDate Table: Universidad Nacional de Ancash "Santiago Antunez de Mayolo"Talía Matta ValverdeNo ratings yet
- Heat Balance Calculation Sheet (For Deaerator)Document1 pageHeat Balance Calculation Sheet (For Deaerator)Hoang Mai HoaNo ratings yet
- Specific Heat of MetalDocument6 pagesSpecific Heat of MetalJohn ChenNo ratings yet
- NG CalculationDocument9 pagesNG CalculationAhmedNo ratings yet
- Cre Ex-5.Document1 pageCre Ex-5.210170105005No ratings yet
- PfeeDocument1,065 pagesPfeeKhalil Ben MansourNo ratings yet
- H-068 Thermo. Titr. Application Note No.: Title: Determination of Ferric Ion by Iodometric TitrationDocument3 pagesH-068 Thermo. Titr. Application Note No.: Title: Determination of Ferric Ion by Iodometric TitrationEko Setyo BudiNo ratings yet
- Production of Butadiene Chapter 1 ProcesDocument68 pagesProduction of Butadiene Chapter 1 ProcesTushar GoyalNo ratings yet
- Monica Gallegos Alor PDFDocument1 pageMonica Gallegos Alor PDFCesar Alberto Cansino PerezNo ratings yet
- P Tables 2pagsDocument2 pagesP Tables 2pagsandres_old_condeNo ratings yet
- BE Samples Water AnalysisDocument2 pagesBE Samples Water AnalysisMohsin ModiNo ratings yet
- N HCL: 1. Metode LangmuirDocument11 pagesN HCL: 1. Metode LangmuirReni MarlinaNo ratings yet
- Your Formula or Analysis NameDocument4 pagesYour Formula or Analysis NamerandolfbangayNo ratings yet
- PVT ExcelDocument8 pagesPVT ExcelolaseyeNo ratings yet
- Ilide - Info Hydrolysis of Methyl Acetate PRDocument3 pagesIlide - Info Hydrolysis of Methyl Acetate PRAlester CuyosNo ratings yet
- Hex 1201 FD LH 16112018Document2 pagesHex 1201 FD LH 16112018Tino FebriantoNo ratings yet
- UntitledDocument6 pagesUntitledMartin BishwasNo ratings yet
- B1. Compressor - Accessories - B2. Ariel Gas Analysis DataDocument4 pagesB1. Compressor - Accessories - B2. Ariel Gas Analysis Dataargasi.mrysiNo ratings yet
- 1-Mix Design UHPGC (W-B (0.35) (Ca 0.3) - Effect of MolarityDocument93 pages1-Mix Design UHPGC (W-B (0.35) (Ca 0.3) - Effect of MolaritySawa Zayia MichaelNo ratings yet
- Natural Gas Density CalculatorDocument3 pagesNatural Gas Density CalculatorVaibhav KuraleNo ratings yet
- Cirstalizacion 2y9 (Recuperado Automáticamente)Document11 pagesCirstalizacion 2y9 (Recuperado Automáticamente)zs22021374No ratings yet
- Lab Four ExerciseDocument3 pagesLab Four ExerciseJeremiahNo ratings yet
- Reaksi Kimia LimestoneDocument6 pagesReaksi Kimia LimestoneRifka FadillahNo ratings yet
- Material Balance: Engineering Services by KBR Technical Services, IncDocument3 pagesMaterial Balance: Engineering Services by KBR Technical Services, IncSanju ChauhanNo ratings yet
- StreamsDocument2 pagesStreamsBurak PolatNo ratings yet
- Oriental Aquamarine Biotech India Oriental Aquamarine Biotech India (P) Limited CoimbatoreDocument35 pagesOriental Aquamarine Biotech India Oriental Aquamarine Biotech India (P) Limited CoimbatorevlabdulazeemNo ratings yet
- Leaching Test Data SheetDocument3 pagesLeaching Test Data SheetDennis Vargas GómezNo ratings yet
- Raw Materials SpecificationsDocument2 pagesRaw Materials SpecificationsSaravanan A100% (1)
- Balance de Materia 1Document12 pagesBalance de Materia 1diego veyzagaNo ratings yet
- Tugas Khusus Ammonia Primary Reformer (Aktual) Tugas KhususDocument224 pagesTugas Khusus Ammonia Primary Reformer (Aktual) Tugas KhususRafi Theda PrabawaNo ratings yet
- HMB 1Document3 pagesHMB 1Besan LaduNo ratings yet
- Nitrowblower 030#1Document27 pagesNitrowblower 030#1ajaymanhasNo ratings yet
- PFL Slow Filter Sand: Chemical AnalysisDocument1 pagePFL Slow Filter Sand: Chemical AnalysisforuzzNo ratings yet
- Group 4 Titration CurveDocument3 pagesGroup 4 Titration CurveEllah GutierrezNo ratings yet
- Mass Balance OnlyDocument29 pagesMass Balance OnlyAzzah Dyah PramataNo ratings yet
- STEAMGEN - PED - Calculations PDFDocument177 pagesSTEAMGEN - PED - Calculations PDFzoran cukovicNo ratings yet
- FT 01, DB ViewDocument6 pagesFT 01, DB ViewZakyAlFatonyNo ratings yet
- Flare Ko Drum Sizing by Api 521 SWP008: 'File:///conversion/tmp/scratch/480402124.xls'#$sheet1Document1 pageFlare Ko Drum Sizing by Api 521 SWP008: 'File:///conversion/tmp/scratch/480402124.xls'#$sheet1Cast Ed IvNo ratings yet
- Validation FolderDocument2 pagesValidation FolderPriyabrata RoyNo ratings yet
- OT 15 MarzoDocument163 pagesOT 15 Marzod1lf1x6hy9No ratings yet
- Ammonia Electrolysis ReportDocument9 pagesAmmonia Electrolysis ReportMuhammad HumzaNo ratings yet
- Fugacidade - Termodinmica IIDocument5 pagesFugacidade - Termodinmica IIGiovanna BolinaNo ratings yet
- Effect of Reinforcement Corrosion On Bond StrengthDocument7 pagesEffect of Reinforcement Corrosion On Bond Strengthdebabrata duttaNo ratings yet
- Acid - Base Reactions Promaths Online Grade 12 Learner MaterialDocument21 pagesAcid - Base Reactions Promaths Online Grade 12 Learner MaterialsihledisebomotaungNo ratings yet
- Multifinger Imaging Tool MITDocument2 pagesMultifinger Imaging Tool MITWaleed Barakat MariaNo ratings yet
- Revision Notes Class 12 Chemistry Chapter 9 - Coordination Compounds Notes in 30 MinutesDocument42 pagesRevision Notes Class 12 Chemistry Chapter 9 - Coordination Compounds Notes in 30 Minutessangee chandranNo ratings yet
- Chapter 13: Oscillations: Energy Conservation in Simple Harmonic OscillationsDocument3 pagesChapter 13: Oscillations: Energy Conservation in Simple Harmonic OscillationsCloudlinNo ratings yet
- Tugas Teknik Pengawetan Dan Emerging Processing Dalam Pengolahan PanganDocument5 pagesTugas Teknik Pengawetan Dan Emerging Processing Dalam Pengolahan PanganHasnaNo ratings yet
- Hydril - GK BopDocument66 pagesHydril - GK Bopnkk1790% (10)
- General Chemistry 1 First SemDocument42 pagesGeneral Chemistry 1 First SemJosie JavierNo ratings yet
- 17PS1ADocument2 pages17PS1ASeamus AlaricNo ratings yet
- Hard WaterDocument11 pagesHard WaterJonathan OtadoraNo ratings yet
- Basic Conservation Laws-CurrieDocument12 pagesBasic Conservation Laws-Currieing_tytyNo ratings yet
- NF 16101 ExplainedDocument8 pagesNF 16101 ExplainedMauricio CairoNo ratings yet
- Statement of Grades: Subject NameDocument13 pagesStatement of Grades: Subject NameAnkit KumarNo ratings yet
- Xii Neet FRGT-02 - Key and Solutions (09.04.23)Document8 pagesXii Neet FRGT-02 - Key and Solutions (09.04.23)Elamparithi ANo ratings yet
- CPT - Lecture 1920 - Sulfuric Acid ProcessDocument29 pagesCPT - Lecture 1920 - Sulfuric Acid ProcessShubham ChoudharyNo ratings yet
- Engleski-Seminarski RadDocument18 pagesEngleski-Seminarski RadMarkoNo ratings yet
- Study Plan: Academic BackgroundDocument2 pagesStudy Plan: Academic BackgroundGhulam MurtazaNo ratings yet
- Chemical Reactions TallerDocument8 pagesChemical Reactions Tallerllamame_palaNo ratings yet
- Applied Chemistry-Tutorial-3Document2 pagesApplied Chemistry-Tutorial-3BigNo ratings yet
- 316 LSDocument5 pages316 LSRouse ToxquiNo ratings yet
- 214.039 Operation of Sieve Shaker2012Document3 pages214.039 Operation of Sieve Shaker2012Pradeep KumarNo ratings yet
- Mold Design - Injection MoldingDocument4 pagesMold Design - Injection Moldinganil chejaraNo ratings yet
- INS - Adjuvants For Enhancing Herbicide PerformanceDocument15 pagesINS - Adjuvants For Enhancing Herbicide PerformanceAlejandro Herrnsdorf-SakellaridisNo ratings yet
- Flexibility Consistent Deformation MethodDocument2 pagesFlexibility Consistent Deformation MethodBia MughalNo ratings yet
- Water Vapour Barrier Beeswax PDFDocument12 pagesWater Vapour Barrier Beeswax PDFElkyn BohórquezNo ratings yet
- Bacterial Enumeration: Standard Plate Count (Viable Counts)Document7 pagesBacterial Enumeration: Standard Plate Count (Viable Counts)Meshal NoorNo ratings yet
- Alcohols, Phenols and Ethers 20 Years Specialized Pyq by Garima GoelDocument14 pagesAlcohols, Phenols and Ethers 20 Years Specialized Pyq by Garima Goeltanishadawar80No ratings yet
- SA-784-A-80875 - HTS-320 MSDS (Hardener) (764123)Document7 pagesSA-784-A-80875 - HTS-320 MSDS (Hardener) (764123)Tomy Efraim SitumorangNo ratings yet