Lab Manual - PRPC
Uploaded by
Kaushal BaldhaLab Manual - PRPC
Uploaded by
Kaushal BaldhaDepartment of Chemical Engineering
Experiment No. 1
Determination of viscosity by Saybolt Viscometer
AIM: -
To determine the viscosity (in Saybolt Universal Seconds) of a liquid hydrocarbon and also
the effect of temperature on the viscosity.
PRINCIPLE
The absolute viscosity of fluid oil can be determined by measuring the rate of flow of the oil
through a capillary tube kept at a uniform temperature. But in case of lubricating oils specific
viscosity is generally determined by measuring the time taken for a given quantity of oil to
flow through an orifice or jet of standard dimension under standard conditions. Three types
of standard viscometers namely Redwood, Engler and Saybolt viscometer are of common
use.
This test method covers the empirical procedures for determining the Saybolt Universal or
Saybolt Furol viscosities of petroleum products at specified temperatures between 21 and
99°C (70 and 210°F). It is referred to as the Saybolt viscosity and written as Saybolt second
universal (SSU). The dynamic viscosity can be obtained by multiplying the kinematic
viscosity by the density of the liquid.
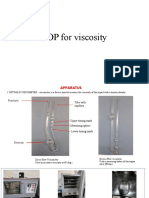
APPARATUS
PROCEDURE
Clean and dry the oil cylinder of viscometer. Insert the cork tightly. Fill the
cylinder up to mark with oil.
Updated on 1st January 2021 by Dr. Kaushik Nath Page 1
Department of Chemical Engineering
Take out excess oil with the help of pipette. Insert a thermometer both in oil bath
and in the paraffin bath.
Start heating at a controlled rate and stir the oil and paraffin bath continuously for
uniform heating.
As the oil sample comes at desired temperature open the cork at bottom and note
the seconds for collection of 60 ml oil at specified temperature.
Repeat the process for a constant reading. Find out a constant flow time at
different temperatures
EXPERIMENTAL RESULTS
Room Temperature:
Temperature of water bath
Collection time Mean time
Volume collection Sr. No
(s) (s)
60 ml. 1
2
3
Plot a graph of flow time versus Kinematic viscosity
Updated on 1st January 2021 by Dr. Kaushik Nath Page 2
Department of Chemical Engineering
The following formula may be used to convert centistokes (cSt) units to approximate Saybolt
universal seconds unit.
For SUS values between 32 and 100
195
cSt 0.226 SUS
SUS
For SUS values greater than 100
135
cSt 0.220 SUS
SUS
Also refer the attached conversion table of centistokes, stokes and SSU.
The viscosity of a given sample using Saybolt viscometer should be denoted with
Saybolt seconds at the specified temperature.
Repeatability of the experiments should be duly compared at low and high
temperatures.
Convert the Saybolt seconds to cSt with the empirical formulation 1cSt as 31 Saybolt
seconds and compare with standard values.
INDUSTRIAL APPLICATIONS
This is used for the empirical measurement of Saybolt Viscoisty of petroleum products at
specified temperatures between 07° F and 210°F. This is also used for determining the
Saybolt Furol Viscosity of bituminous materials at temperature of 250, 275, 300, 350, 400
and 450° F.
DISCUSSIONS
The result obtained from this test method is dependent upon the behavior of the sample and is
intended for application to liquids for which primarily the shear stress and shear rates are
proportional (Newtonian flow behavior). If, however, the viscosity varies significantly with
the rate of shear, different results may be obtained from viscometers of different capillary
diameters. The range of kinematic viscosities covered by this test method is from 0.2 to 300
000 mm²/s at all temperatures.
SHORT QUESTIONS
1. How does the viscosity of gas/liquid vary with temperature?
2. Define kinematic viscosity?
3. How do you improve the viscosity index of lubricating oil?
4. How viscosity is expressed in Saybolt Viscometer?
5. Name a few other viscometers for testing of viscosities of lube oils.
6. Name a few lubricants with high viscosity index.
Updated on 1st January 2021 by Dr. Kaushik Nath Page 3
Department of Chemical Engineering
Experiment No. 2
Determination of Flash Point by Pensky Marten Apparatus
AIM
To find out the Flash point of fuel oils or lubricating oils ranging above 49oC by Pensky
Marten Close cup apparatus.
PRINCIPLE
Flash point is defined as the temperature at which an oil gives sufficient vapor to form an
inflammable mixture with air and catches fire momentarily flashes when flame is applied.
Flash point gives the idea about the nature of the boiling point diagram of the system,
amount of low boiling fraction present in the liquid fuel, explosion hazards, volatility of
the liquid fuels. Beside oil‟s volatility and inflammability limits of the vapor-air mixture
the flash point also depends on the design of the apparatus, the test procedure and
barometric pressure.
APPARATUS
There are two basic types of flash point measurement: open cup and closed cup. In open
cup devices the sample is contained in an open cup (hence the name) which is heated, and
at intervals a flame is brought over the surface. The measured flash point will actually
vary with the height of the flame above the liquid surface, and at sufficient height the
measured flash point temperature will coincide with the fire point. Examples include
Cleveland Open Cup and Pensky-Martens open cup. The main difference being that the
former is heated from below, while the later is heated from the sides as well as below.
Closed cup testers, of which the Pensky-Martens closed cup is one example, are sealed
with a lid through which the ignition source can be introduced periodically. The vapour
above the liquid is assumed to be in reasonable equilibrium with the liquid.
The apparatus consists of the following:
(1) Cast iron bath provided with bras cup cover
(2) Flame arrangement
(3) Shutter mechanism
(4) Hand operated stirrer
Updated on 1st January 2021 by Dr. Kaushik Nath Page 4
Department of Chemical Engineering
Pensky-Marten Flash Point Apparatus
PROCEDURE
Clean and dry the brass cup. Fill the cup with sample exactly to the mark. Adjust the
micro flame. Heat the cup slowly at a controlled rate of 2oC per minute. Pass the test
flame across the center of the cup at every degree in temperature rise. Record the lowest
temperature at which flame is observed at any point over the liquid.
OBSERVATION TABLE
Room Temperature:
Atmospheric pressure:
Sample Given:
Characteristics of the sample:
Sample Serial Number Temperature (oC) Observation
Updated on 1st January 2021 by Dr. Kaushik Nath Page 5
Department of Chemical Engineering
Observed Flash Point: --------------- oC
Since the pressure varies from place to place there is a pressure correction required to
be applied. The following correlation is used:
Δtp = 0.0346 (760P) oC
Where P = atmospheric pressure under test conditions.
Actual Flash point = Observed flash point + Pressure correction factor.
TASK
1) Draw the profile of time vs. temperature.
2) Compare the results with the literature value.
3) Can the experiment be conducted to determine the fire point?
INDUSTRIAL APPLICATION
Flash and fire point are important when oil is exposed to high temperature service.
This test provides safe guard against decomposition and fire hazard during
storage, transportation, handling and other uses.
Flash point is used to assess the overall hazard of a material and is used in
shipping and safety regulations to define "flammable" and "combustible"
materials.
DISCUSSION
The flash point is an empirical measurement rather than a fundamental physical
parameter. The measured value will vary with equipment and test protocol variations,
including temperature ramp rate (in automated testers), time allowed for the sample to
equilibrate, sample volume and whether the sample is stirred.
Methods for determining the flash point of a liquid are specified in many standards. For
example, testing by the Pensky-Martens closed cup method is detailed in ASTM D93,
IP34, ISO 2719, DIN 51758, JIS K2265 and AFNOR M07-019. Determination of flash
point by the Closed Cup Equilibrium method is specified in ISO 1523:2002.
REFERENCE
I.S 1448 – 1970 Methods of test for petroleum and its products (P: 21) Flash Point(
Closed) by Pensky Martin apparatus.)
Updated on 1st January 2021 by Dr. Kaushik Nath Page 6
Department of Chemical Engineering
SHORT QUESTIONS
1. Define Flash point and Fire point of petroleum oil.
2. What are the factors that affect flash and fire point?
3. What should be the flash point of a good lubricant?
4. What is the difference between Abel‟s and Pensky Marten‟s Apparatus?
5. What is the permissible flash point value of marketable kerosene?
6. Arrange the following fuels in order of increasing flash point
Kerosene, Gasoline, Diesel, lube oil
7. Is there any relationship between flash point and IBP of oil?
8. What is the significance of flash point determination of petroleum oil?
Updated on 1st January 2021 by Dr. Kaushik Nath Page 7
Department of Chemical Engineering
Experiment No: 3
Determination of Aniline point of petroleum oil
AIM
To determine the aniline point of the supplied oil samples using aniline point apparatus
and to find out the diesel index number of the diesel oil.
APPARATUS & CHEMICALS
Two micro burettes, Test tube, Thermometer, Heating bath, Aniline, Kerosene, Diesel,
Ethyl alcohol / Acetone.
PRINCIPLE
The aniline point is called the "aniline point temperature," which is the lowest
temperature (°F or °C) at which equal volumes of aniline (C6H5NH2) and the oil form a
single phase. The aniline point (AP) correlates roughly with the amount and type of
aromatic hydrocarbons in an oil sample. A low AP is indicative of higher aromatics,
while a high AP is indicative of lower aromatics content.
Aniline point is defined as the minimum temperature at which equal volumes of
anhydrous aniline and oil mix together.
Diesel index, (I.P 21/I.S P: 17) a derivative of aniline point is defined as
[0.018 A.P oC + 0.32] API
Aniline being an aromatic compound freely mixes with aromatics; so a low aniline point
indicates low diesel index, because of high percentage of aromatics.
PROCEDURE
Wash the micro burettes and beakers with ethyl alcohol/acetone.
Take 5ml of aniline and diesel/kerosene to each of the two burettes and
hold in stands.
Collect two mixers in a test tube. Heat it by placing in a water bath, with a
thermometer kept inside.
Heat the test tube with continuous shaking.
Updated on 1st January 2021 by Dr. Kaushik Nath Page 8
Department of Chemical Engineering
After some time you will find that the layers will disappear and form
turbidity.
Then the mixture was cooled and at some instant it was noticed that the
layer again appeared. Temperature at this point was noted.
The same procedure was applied for diesel but for petrol the mixture was
cooled in an ice bath and aniline point was found out by taking it out of ice
bath.
Take the reading in 0C , then convert in to 0F
OBSERVATION TABLE
Volume Volume Average Average
Aniline Aniline Aniline
of of oil Aniline Aniline
Oil sample point 0C point 0C point 0C
aniline sample point in point in
1 2 3 0 0
(ml) (ml) C F
Kerosene
Diesel
Petrol
DIESEL INDEX
RESULTS
DISCUSSION
INDUSTRIAL APPLICATION
Aniline point is used to characterize pure hydrocarbons and to indicate the
aromatic content of hydrocarbon mixtures.
The value gives an indication of the aromatic content of diesel oil, since aniline is
an aromatic compound, which is dissolved on heating by the aromatics in diesel
oil. The greater the aniline point, the lower the aromatics in diesel oil. A lower
aniline point also indicates a higher proportion of paraffin.
A higher aniline point (and therefore a lower aromatic content) in diesel oil is
desirable, in order to prevent auto ignition in diesel engines.
The auto ignition temperature or kindling point of a substance is the lowest
temperature at which it will spontaneously ignite in a normal atmosphere without
Updated on 1st January 2021 by Dr. Kaushik Nath Page 9
Department of Chemical Engineering
an external source of ignition, such as a flame or spark. This temperature is
required to supply the activation energy needed for combustion. The temperature
at which a chemical will ignite decreases as the pressure increases or oxygen
concentration increases. It is usually applied to a combustible fuel mixture.
PRECAUTIONS
The whole apparatus and all the reagents must be perfectly dry as the presence of
even traces of moisture shows high results.
Aniline being hygroscopic so the test tube used must be dried.
Aniline being highly toxic the care should be observed while sucking it through
pipette and perfectly it should be taken with a pipette provided with a rubber
suction bulb.
EXERCISE
1. Mention the formula for Deg. API & characterization factor and explain their
significance.
2. Define Aniline point & explain its significance.
3. Why Aniline has been selected as a solvent for the determination of aniline point?
4. Is there any similarity between aniline point and smoke point?
5. What is Diesel index?
6. Define Cetane number and give its significance.
7. Define Octane number and give its significance.
8. Name the various antiknocking agents with their importance.
9. Which types of oil have the highest aniline point?
10. How is aniline point related to the ignition quality of a diesel fuel?
Updated on 1st January 2021 by Dr. Kaushik Nath Page 10
Department of Chemical Engineering
Experiment No. 4
Determination of viscosity by Redwood Viscometer
AIM
To determine the viscosity (in „Redwood seconds‟) of a liquid hydrocarbon and also the
effect of temperature on the viscosity.
PRINCIPLE
The absolute viscosity of a fluid oil can be determined by measuring the rate of flow of the
oil through a capillary tube kept at a uniform temperature. But in case of lubricating oils
specific viscosity is generally determined by measuring the time taken for a given quantity of
oil to flow through an orifice or jet of standard dimension under standard conditions. Three
types of standard viscometers namely Redwood, Engler and Saybolt viscometer are of
common use.
The viscosity of a hydrocarbon can be expressed as the number of seconds taken for the
collection of 50ml. of the liquid when flowing under standard conditions through a jet of
standard dimensions. The equipment specified is the Redwood Viscometer. The Redwood
apparatus measures viscosity in empirical units and not in absolute unit such as centistoke.
These viscometers are designed for viscosity tests of petroleum Products. They confirm to
requirements of IP 70
APPARATUS
The viscometer consists of an oil cup furnished with a pointer, which ensures a constant head
of oil, and an agate jet, which is drilled with a central hole. The upper end of the agate jet is
closed with a ball, which is lifted to allow the flow of oil during the experiment. The outer
jacket which is for maintaining the oil at a constant temperature is electrically heated and
normally contains water, though if a higher temperature is required, cylinder oil is used. The
temperature is maintained at a uniform level by rotating the stirrer. A wire stirrer is also
provided for mixing the oil samples.
Viscometer No.1 or No.2 is used depending on whether the time of flow of the oil at the
desired temperature, is greater or less than 2000 seconds. The difference between the two
viscometers is the diameter of the orifice.
Redwood No. 1 Capillary diameter.1.62 mm, length. 10.0 mm
Redwood No. 2 Capillary diameter. 3.5 mm, length. 5.0 mm
Updated on 1st January 2021 by Dr. Kaushik Nath Page 11
Department of Chemical Engineering
This means there is a factor of ten between the two viscometers, i.e. a liquid that takes
100 seconds to flow through a No1 viscometer will take 10 seconds in a No2 viscometer.
For oil samples with Redwood seconds less than 2000, Redwood No.1 is
recommended. For highly viscous fluids with greater than 2000 Redwood seconds,
Redwood No.2 is recommended.
Redwood Viscometer
PROCEDURE
The sample of oil, filtered if necessary, is transferred to the container cup of the
viscometer until the pointer just breaks the surface of the oil. It is most
important that the test is always started with the pointer just emerging through
the liquid surface. This ensures that the head of oil above the orifice is always the
same and therefore that the pressure forcing the oil through is the same in each
test using the same oil.
Whilst slowly rotating the jacket stirrer, the water in the jacket is heated to a
temperature of 41oC. When the sample of oil in the cup reaches 40oC., the ball is
lifted and at the same time a stop-watch is started.
Allow oil to run through into the collecting flask until the 50 ml mark on the flask
is reached, at which point the clock should be stopped and the ball pushed back
onto the orifice to halt the oil flow.
The time taken for the passage of 50 ml of oil through the jet into the collecting
flask, in seconds, should be noted. For Redwood 1 do three tests per sample and
for the Redwood 2 do one test per sample. Determination of viscosity in this way
Updated on 1st January 2021 by Dr. Kaushik Nath Page 12
Department of Chemical Engineering
should be made at the following temperatures:- 30oC, 40oC, 60oC, 90oC. The
temperatures suggested do not have to be exactly these values but you must
record the actual values used accurately.
EXPERIMENTAL RESULTS
Room Temperature:
Time Required to Mean
Temperature Kinematic
Sr. No o collect 50 ml of oil Time
C viscosity
(s) (s)
RESULTS
The viscosity of an oil sample with the help of Redwood Viscometer at ----oC is ----------
- Redwood seconds
Updated on 1st January 2021 by Dr. Kaushik Nath Page 13
Department of Chemical Engineering
Redwood viscosity obtained as above can be converted in to kinematic viscosity by using
formula given below:
V = At B/t; where V = kinematic viscosity of the oil in centistokes.
t = time of flow (efflux time) in seconds
A & B are instrument constant
(A) = 0.264 and (B) = 190, when t = 40 to 85 seconds
(A) = 0.247 and (B) = 65, when t = 85 to 2000 seconds.
100 centistokes = 1 stoke
Unit conversions: Stoke Density = Poise (Absolute density)
1/Poise = Centipoise
Kinematic viscosity = Viscosity absolute/Sp. Gravity
The viscosity of a given sample using Redwood viscometer should be denoted with
Redwood seconds at the specified temperature.
b) Repeatability of the experiments should be duly compared at low and high
temperatures.
c) Convert the Redwood seconds to cSt with the empirical formulation 100 Redwood
seconds as 24.5cSt and compare with the standard values.
INDUSTRIAL APPLICATIONS
Redwood viscometer apparatus are widely used in Petroleum Laboratories, Industries, Oil
Refineries, Educational Institutions, Research Organizations for standardization and
determines the Viscosity of Petroleum products, which flows in a Newtonian liquid
except cut back Bitumens and road oils at the set temperature. They confirm to
requirement of IP 70.
SHORT QUESTIONS:
1. How does the viscosity of liquid vary with temperature?
2. Define viscosity. Mention its units in various systems.
3. What is viscosity index?
4. How do you improve the viscosity index of lubricating oil?
5. What is the difference between the Redwood Viscometer No 1 and Redwood No.
2?
6. Name a few lubricants with high viscosity index.
Updated on 1st January 2021 by Dr. Kaushik Nath Page 14
Department of Chemical Engineering
Difference between Redwood and Saybolt Viscometer
Sr. No. Saybolt Viscometer Redwood Viscometer
1 The experiment is held and a 60ml of oil In this experiment is held and a
is passed through a standard orifice. The 50ml of oil is passed through a
total time duration is then noted. standard orifice. The total time
duration is then noted.
2 A stopper is provided at the bottom of The stopper is replaced with a ball
the tube for the liquid not to flow. It is valve and orifice.
released during the experiment.
Updated on 1st January 2021 by Dr. Kaushik Nath Page 15
Department of Chemical Engineering
Experiment No. 5
Determination of Flash Point by Abel’s Apparatus
AIM
To find out the Flash point of petroleum products and mixtures above 19oC and below
70oC ranging by Abel‟ apparatus (as per IP: 33 and 1448 [part 1, p.20], 1982).
PRINCIPLE:
Flash point is defined as the temperature at which an oil gives sufficient vapor to form an
inflammable mixture with air and catches fire momentarily flashes when flame is applied.
Flash point gives the idea about the nature of the boiling point diagram of the system,
amount of low boiling fraction present in the liquid fuel, explosion hazards, volatility of
the liquid fuels. Beside oil‟s volatility and inflammability limits of the vapor-air mixture
the flash point also depends on the design of the apparatus, the test procedure and
barometric pressure.
APPARATUS
There are two basic types of flash point measurement: open cup and closed cup. In open
cup devices the sample is contained in an open cup (hence the name) which is heated, and
at intervals a flame is brought over the surface. The measured flash point will actually
vary with the height of the flame above the liquid surface, and at sufficient height the
measured flash point temperature will coincide with the fire point.
The apparatus is made of brass /gun metal machined cup cover fitted with shutter
mechanism, test flame arrangement, stirrer and thermometer socket. The total assembly
of the apparatus rests in stainless double jacketed copper/stainless steel water bath. Other
components like a funnel, an overflow pipe, and split thermometer socket are fixed on the
top of the heating bath and its outer jacket of the bath is fitted with a stand. At the bottom
of the apparatus, an electric heater is fitted with a flexible cord.
PROCEDURE
The test portion is placed in the test cup of an Abel apparatus and heated to give a
constant temperature increase with continuous stirring.
A small test flame is directed through an opening in the test cup cover at regular
temperature intervals with simultaneous interruption of stirring.
Updated on 1st January 2021 by Dr. Kaushik Nath Page 16
Department of Chemical Engineering
The lowest temperature at which application of the test flame causes the vapour of
test portion to ignite and propagate over the surface of the liquid is recorded as the
flash point at the ambient barometric pressure.
The temperature is corrected to standard atmospheric pressure using an equation.
Separate test procedures are defined for liquids with expected flash points
between –30 °C and 18,5 °C inclusive, and between 19 °C and 70 °C inclusive.
Abel’s Flash Point Apparatus: Assembly plus heating Vessel
Updated on 1st January 2021 by Dr. Kaushik Nath Page 17
Department of Chemical Engineering
OBSERVATION TABLE
Room Temperature:
Atmospheric pressure:
Sample Given:
Characteristics of the sample:
Sample Serial Number Temperature (oC) Observation
Observed Flash Point: --------------- oC
Since the pressure varies from place to place there is a pressure correction required to
be applied. The following correlation is used:
Δtp = 0.0346 (760 P) oC
Where P = atmospheric pressure under test conditions.
Actual Flash point = Observed flash point + Pressure correction factor.
TASK
4) Draw the profile of time vs. temperature.
5) Compare the results with the literature value.
6) Can the experiment be conducted to determine the fire point?
INDUSTRIAL APPLICATION
Updated on 1st January 2021 by Dr. Kaushik Nath Page 18
Department of Chemical Engineering
Flash and fire point are important when oil is exposed to high temperature service.
This test provides safe guard against decomposition and fire hazard during
storage, transportation, handling and other uses.
Flash point is used to assess the overall hazard of a material and is used in
shipping and safety regulations to define "flammable" and "combustible"
materials.
DISCUSSION
The flash point is an empirical measurement rather than a fundamental physical
parameter. The measured value will vary with equipment and test protocol variations,
including temperature ramp rate (in automated testers), time allowed for the sample to
equilibrate, sample volume and whether the sample is stirred.
Flash point values may be used in shipping, storage, handling and safety regulations, as a
classification property to define “flammable” and “combustible” materials. Precise
definition of the classes is given in each particular regulation. A flash point value may
indicate the presence of highly volatile material(s) in a relatively non-volatile or
nonflammable material and flash point testing may be a preliminary step to other
investigations into the composition of unknown materials.
SHORT QUESTIONS
1. Define Flash point and Fire point of petroleum oil.
2. What are the factors that affect flash and fire point?
3. What should be the flash point of a good lubricant?
4. What is the difference between Abel‟s and Pensky Marten‟s Apparatus?
5. What is the permissible flash point value of marketable kerosene/diesel/gasoline?
6. Arrange the following fuels in order of increasing flash point
a. Kerosene, Gasoline, Diesel, lube oil
7. Is there any relationship between flash point and IBP of oil?
9. What is the significance of flash point determination of petroleum oil?
Updated on 1st January 2021 by Dr. Kaushik Nath Page 19
Department of Chemical Engineering
Experiment No. 6
Determination of Carbon residue by Conradson carbon Apparatus
AIM
To find out the carbon residue of supplied sample of fuel oil using Conradson carbon
apparatus (As per IP-13 & ASTM D 189).
PRINCIPLE
Carbon residue of oil is defined as percentage of carbon left after complete evaporation
and pyrolysis of the oil taken, heated under standard condition in a Conradson‟s
apparatus. The result gives information about relative carbon forming property of oil
which is useful for its use as a lubricant. The carbon residue of a crude oil product gives
an indication of the propensity for that product to form a carbonaceous residue under
thermal conditions. The carbonaceous residue is correctly referred to as the carbon
residue but is also often referred to as coke or thermal coke.
Carbon residue is an indication of the fuel to decompose and form carbonaceous
material that can plug diesel fuel injection nozzles. The carbon residue provides
information on the carbonaceous deposits which will result from combustion of the fuel.
For fuels with a high carbon- high carbon/hydrogen ratio, it is proved more difficult to
burn them fully, which results in increased deposits in the combustion and exhaust
spaces. Fuels with a high carbon residue value may cause problems in older engines
when they are operating under part load conditions. The carbon residue value of a fuel
depends on the refinery processes employed in its manufacture.
Ramsbottom Carbon Residue (RCR), Conradson Carbon Residue (CCR), Micro
Carbon Residue (MCR) are the most commonly used indexes for estimation of carbon
residue.
APPARATUS
It consists of the following:
i) Porcelain crucible
ii) Iron crucible (skidmore)
iii) Spun sheet iron crucible
iv) Hood
v) Burner
Updated on 1st January 2021 by Dr. Kaushik Nath Page 20
Department of Chemical Engineering
The Conradson apparatus mainly consists of three crucibles kept one inside other. The
inner most is a silica crucible which consists of the oil along with two glass beads. This is
provided with a lid, having a small tube type opening for the escape of volatile matter.
The combination is then placed inside a spun sheet crucible covered with a chimney
shaped iron hood.
PROCEDURE
About 1 gm of oil under test is to be taken in a porcelain or silica crucible with one or
two of glass beads to prevent bumping during heating. The crucible is then placed in a
skidmore iron crucible of capacity 65-82 ml, containing sand so that the levels of the two
crucibles are same. Initially it is to be heated strongly so that ignition starts within 10±1
min, marking the pre-ignition period. When smoke appears over the chimney, burner is to
be switched on so that the sides of crucible are heated and vapors are formed. The flame
is adjusted so that the vapors burn uniformly and the burning period is 13 ± 1min when
the vapor ceases to burn and no more blue smoke is observed, the burner is readjusted to
heat at the bottom as in beginning and heating is continued to red hot for exactly 7 min.
Thus the total heating period is 30 ± 2 min. After heating the arrangement is cooled until
no smoke appears. The hood is then removed and silica crucible is cooled is desiccator
and weighed.
Updated on 1st January 2021 by Dr. Kaushik Nath Page 21
Department of Chemical Engineering
OBSERVATIONS
Weight of the crucible with glass beads = __________
Weight of crucible + Glass bead + Oil = _______________
Weight of crucible with glass bead and residue after cooling = _______________
% age of Carbon residue in oil = (weight of Carbon residue/weight of oil taken) ×
100.
RESULT
TASK
Compare the results with literature value.
INDUSTRIAL APPLICATION
Propensity of thermal cracking in petroleum refining is an outright function of Conradson
carbon residue (CCR). Industrially a concise analogical term Conradson Decarbonizing
Efficiency (CDE) is more familiar; it is given by
carbon residue in feedstock carbon residue in liq products
CDE
Carbon residue in feedstock
The carbon residue value of burner fuel serves as a rough approximation of the
tendency of the fuel to form deposits in vaporizing pot-type and sleeve-type
burners. Similarly, provided alkyl nitrates are absent (or if present, provided the
test is performed on the base fuel without additive) the carbon residue of diesel
fuel correlates approximately with combustion chamber deposits.
The carbon residue value of motor oil, while at one time regarded as indicative of
the amount of carbonaceous deposits a motor oil would form in the combustion
chamber of an engine, is now considered to be of doubtful significance due to the
presence of additives in many oils. For example, an ash-forming detergent
additive may increase the carbon residue value of oil yet will generally reduce its
tendency to form deposits.
The carbon residue value of gas oil is useful as a guide in the manufacture of gas
from gas oil, while carbon residue values of crude oil residuum‟s, cylinder and
bright stocks, are useful in the manufacture of lubricants.
Updated on 1st January 2021 by Dr. Kaushik Nath Page 22
Department of Chemical Engineering
Exercise: -
1. Name the various methods to determine the carbon residue in petroleum fractions.
2. For which fraction carbon residue is an importance specification.
3. What are the constituents of LPG?
4. What is the difference between gross calorific value and net calorific value of
fuel?
5. What are the different types of fuel gases? Give their detail composition and
heating value.
Updated on 1st January 2021 by Dr. Kaushik Nath Page 23
Department of Chemical Engineering
Experiment No. 7
ASTM Distillation
(As per IS – 1448 (Part.1) 1960 (P: 18) and As per IP 18 Specification)
AIM:
This test is intended for the determination of the distillation characteristics of natural,
gasoline, aviation and motor fuel special boiling point spirit, white spirit and petroleum
distillation having volatities intermediate between those of kerosene and lubricating oil.
PRINCIPLE:
This test method covers the determination of the boiling range distribution of petroleum
products. This test method is applicable to petroleum distillates having an initial boiling
point greater than 100°C and a final boiling point less than 615°C at atmospheric pressure
as measured by this test method. ASTM stands for American Society for Testing and
Materials.
Dry point: The temperature indicated by the distillation thermometer when the last drop
of liquid leaves the bottom of the distillation flask.
Final boiling point (FBP): Maximum temperature indicated during the distillation.
Initial boiling point (IBP): The temperature indicated by the distillation thermometer at
the instant the first drop of condensate leaves the end of the condenser tube.
APPARATUS:
The sample is distilled in specified apparatus under prescribed conditions of heat input
and rate of distillation. One hundred milliliters of the sample are distilled in method A
and method B and 200 ml in method C. Temperature are observed on the specified total
immersion thermometer in order to obtain the initial and final boiling points of the
distillation and the dry point, if required. Either the percentage of distillate recovered at
prescribed temperature intervals or the temperatures at which prescribed percentage of
distillate are collected in the receiver are recorded. Correction of the observed
temperatures for incomplete immersion of the thermometer is not made.
Collect the sample in a previously cooled bottle preferably by immersing the bottle in the
liquid and discarding the first sample. When immersion is not possible draw off the
sample into a previously cooled bottle keeping agitation at a minimum. Close the bottle
immediately with a tight stopper and place it in a ice bath or a refrigeration to bring the
sample to a temperature between 0 to 5 oC.
Updated on 1st January 2021 by Dr. Kaushik Nath Page 24
Department of Chemical Engineering
PROCEDURE:
Cool the distillation flask, the receiver and the sample to 0 to 5 oC. Measure 100
ml of the sample into the distillation flask by means of the receiver taking care
that none of the liquid flows into the vapour tube.
Cool the thermometer to between 0 and 5oC and dry it before fitting it tightly into
the flask by means of a cork. Adjust it so that it will be in the middle of the neck
and so that the lower and of the capillary is level with the bottom of the inside of
the vapor tube at its junction with the neck of the flask.
Ensure that the heating apparatus, including the draught screen and flask support
are all at room temperature at the start of each distillation. Place the flask with its
contents on the 32 mm opening the upper asbestos board with the vapour tube
inserted into condenser.
Make the connection tight by means of a cork through which the vapour tube
passes and adjust the position of the flask so that the vapour tube extends into the
condenser not less than 25 mm and not more than 50 mm.
Without drying it, place the graduated receiver used in measuring the charge at
the outlet of the condenser in such a position that the condenser tube extends into
the receiver at least 25 mm but not below the 100 mm mark. Immerse the receiver
up to the level of the outlet of the condenser tube in transparent liquid bath
maintained between the temperatures of 0 to 1 oC. Cover the top of the receiver
closely during the
Updated on 1st January 2021 by Dr. Kaushik Nath Page 25
Department of Chemical Engineering
RESULTS
Room Temperature:-
Observation table: -
Condensate Collected in Temperature of the test
Sr
ml sample in
No. 0
C
1 First drop 54
2 5 56
3 10 58
4 15 60
5 20 62
6 25 64
7 30 66
8 35 70
9 40 72
10 45 79
11 50 88
12 55 104
13 60 118
14 65 126
15 70 136
16 75 142
17 80 150
18 85 159
19 90 172
20 95 230
21 Last drop 260
Graph: -
Temperature of the test sample vs. Condensate Collected (Find the true boiling point and
final boiling point of the sample from graph)
INDUSTRIAL APPLICATIONS
Updated on 1st January 2021 by Dr. Kaushik Nath Page 26
Department of Chemical Engineering
It is generally known in petrochemical industry to perform a distillation analysis
of a hydrocarbon-containing compound such as a petroleum product (e.g. gasoline
and/or naphta) in order to obtain distillation data such as boiling point range and
the like. The boiling range distribution of light and medium petroleum distillate
fractions provides an insight into the composition of feed stocks and products
related to petroleum refining process.
This test method covers the atmospheric distillation of petroleum products using a
laboratory batch distillation unit to determine quantitatively the boiling range
characteristics of such products as light and middle distillates, automotive spark-
ignition engine fuels, automotive spark-ignition engine fuels containing up to 10
% ethanol, aviation gasolines, aviation turbine fuels, 1-D and 2-D diesel fuels,
biodiesel blends up to 20 %, marine fuels, special petroleum spirits, naphthas,
white spirits, kerosene, and Grades 1 and 2 burner fuels.
Exercise: -
1. What do you understand from ASTM distillation and True Boiling Point (TBP)
overlap? Also explain their significance with respect to motor spirit.
2. State any four thermal properties of petroleum fractions.
3. What is the elemental and chemical Composition of petroleum?
4. What are the various types of gases obtained during production and refining of
crude oil?
5. Define the terms cracking, reforming and hydro treating.
6. Name the catalysts used in catalytic cracking and reforming and explain the
mechanisms.
7. What is the purpose of visbreaking process in petroleum refinery?
8. Name the various petroleum refinery products and mention their applications.
9. What do you understand by stabilization of crude oil and how is it carried out?
10. State and discuss the various methods for dehydration of crude.
Updated on 1st January 2021 by Dr. Kaushik Nath Page 27
Department of Chemical Engineering
Experiment No. 8
Determination of Cloud and Pour point of Petroleum oil
AIM:
This test method is intended to determine the cloud and pour point of petroleum oils used
in the softening and stuffing of leather, as well as those used in the manufacture of
products for such purpose. The pour point of petroleum oils is measured for the purpose
of quality assurance. (ASTM D5346 - 93(2003) e1
PRINCIPLE:
Some petroleum fractions, particularly middle distillate petroleum fuels and dark oils
typically contain varying quantities of dissolved wax. Waxes are hydrocarbons of high
molecular weight which are solids at about 70° F. or less such as paraffin and
microcrystalline. Wax has a tendency to crystallize within a petroleum fraction as the
petroleum fraction temperature decreases. Crystallized wax causes the petroleum fraction
to cloud, become viscous and, as the temperature drops, to solidify. The cloud point of a
fuel oil is the temperature at which the fuel becomes hazy or cloudy because of the
appearance of wax crystals. Cloud & pour point are indicators of the lowest temperature
of utility for petroleum products. The sample is periodically examined while it is being
cooled in the cloud & pour point apparatus. The highest temperature at which haziness is
observed (cloud point) or the lowest temperature at which movement of the oil is
observed (pour point) is reported as the test result.
PROCEDURE
If required dry the test oil by shaking it with a small amount of anhydrous sodium
sulphate and filter it through lintless filter paper.
Fill the oil up to the mark inside the flat bottomed glass tube and fit the tube with
a cork in to which a thermometer is inserted. The bulb of the thermometer should
dip inside the oil.
Keep the tube in the cooling air jacket surrounded by the cooling bath containing
the cooling mixture. The temperature of the oil will start falling down.
After every 1oC fall in temperature of oil, take out the test jar from the jacket and
inspect for cloudiness. If no cloudiness appears, immediately replace it in the
jacket.
Updated on 1st January 2021 by Dr. Kaushik Nath Page 28
Department of Chemical Engineering
Record the temperature at which such inspection first reveals a distinct cloudiness
in the oil near the bottom of the jar.
Continue the cooling and take out the tube after every 3 oC fall of temperature and
tilt it just enough to see any movement or flow of oil. If the movement or flow is
there, replace it immediately in the jar.
Continue the test until no movement of oil is observed when the test jar is held in
a horizontal position for exactly 5 seconds. Note down the temperature and report
it as pour point.
OBSERVATION TABLE
Room Temperature:
Sample Given:
Cloud Point:
Pour Point:
Cloud and Pour Point Apparatus
TASK: Collect the pour point values of various lube oils from literature. Compare your
result with literature value.
Updated on 1st January 2021 by Dr. Kaushik Nath Page 29
Department of Chemical Engineering
INDUSTRIAL APPLICATION:
The lowest temperature (in °F or °C) at which a liquid remains pourable (meaning it still
behaves as a fluid). Oil or synthetic muds with high pour points may suffer from poor
screening and excessive pressure, surges in deepwater wells or other operations subject to
low temperatures. In oils, the pour point is generally increased by a high paraffin content.
The pour point of liquid additives is an important consideration for arctic drilling
operations.
DISCUSSION:
A particular problem exists during the winter months when wax crystallization caused by
low outdoor temperatures can cause fuel lines, filters and the like to clog in diesel engines
and furnaces which burn petroleum fractions. Pour point depressants such as kerosene
and polymerized higher esters of acrylic acid derivatives are well known. When added to
a petroleum fraction, pour point depressants lower the cloud point and the pour point
temperature of the petroleum fraction. Kerosene is also used as a diluent to lower the
cloud point of petroleum fractions. However, wax content within a petroleum fraction
often varies with the distillation conditions under which the fraction was produced and
with the source of crude oil from which the fraction was distilled. Thus, the amount of
pour point depressant needed to achieve a desired pour point in a petroleum fraction will
vary.
SHORT QUESTIONS:
1. Define cloud and pour point of a liquid lubricant.
2. How can the pour point of an oil be lowered?
3. What is the significance of pour point and cloud point determination?
4. Name a few pour point depressants.
5. What are the factors for pour point fixation of a fuel?
6. Name a few freezing mixtures used for pour point analysis.
Updated on 1st January 2021 by Dr. Kaushik Nath Page 30
Department of Chemical Engineering
Experiment No. 9
Determination of surface tension of a given liquid by Stalagmometer
AIM: To determine the surface tension of a given liquid at ambient temperature.
PRINCIPLE:
Surface tension is the characteristic property of every liquid and it is due to
intermolecular attractions among molecules of liquid. It is defined as the force in dynes
acting at right angles to the surface of a liquid or a solution along one centimeter length
of surface. The surface tension of a given liquid is determined relative to water at the
room temperature by using stalagmometer. The number of drops for the same volume of
water and the given liquid are counted and let these be as n1 and n2 respectively. Now if
d1 and d2 are densities of water and the given liquid at the room temperature as
determined separately by using pyknometer, then the surface tension 2 of the given
liquid can be calculated by using simplified relationship as:
1 n 1 d1
2 n2 d2
MATERIALS:
Stalagmometer, distilled water, unknown liquid, beaker, rubber tube with screw pinch
cock, clamp stand, pyknometer.
DESCRIPTION OF APPARATUS
The stalagmometer consists of a bulbed dropping tube with a capillary. The lower end of
capillary is well ground, polished and flattened in order to provide large dropping
surface. There are two marks above and below the bulb. The upper end of the apparatus is
connected with a rubber tube with a screw pinch cock in order to control the rate of flow
so that spherical drop may be formed. Hence by simply counting the number of drops for
the given liquid and water and knowing their density, we can find the relative surface
tension of the liquid. The advantages of the drop pipette method are that it is very
convenient and quick. It can also be employed for determination at different temperatures
by placing the whole apparatus in thermostat.
Updated on 1st January 2021 by Dr. Kaushik Nath Page 31
Department of Chemical Engineering
PROCEDURE
Clean the stalagmometer and pyknometer thoroughly first with chromic acid
solution and wash finally with distilled water and then dry.
By immersing the lower end in a beaker containing the distilled water, suck up
water until it rises above the mark and tighten the screw of the screw pinch.
Now loosen the screw of the screw pinch carefully so that the liquid drops
start falling at the interval of about 2-3 seconds in successive drops. Counting
of drops is started when the water meniscus just reaches the upper mark and
stopped when the meniscus just passes the lower mark. Repeat to get three
readings and take the mean value.
Clean the stalagmometer and dry it. Fill it with liquid until it rises above the
upper mark and count the number of drops as before.
OBSERVATINS & CALCULATIONS
Room Temperature = oC
Density of water at room temperature = d1
Weight of empty pyknometer = w1 g
Weight of pyknometer with water = w2 g
Weight of pyknometer with liquid under test (same volume as water) = w2
Weight of water = ( w2-w1) = m1 g (say)
Weight of liquid = (w3-w1) = m2 g (say)
Updated on 1st January 2021 by Dr. Kaushik Nath Page 32
Department of Chemical Engineering
Surface tension of water at room temperature =
Liquid Number of drops Mean value
(1) (2) (3)
n1
n2
n3
CALCULATIONS
Density of the given liquid relative to water at room temperature
m1
d2
m2
n d
Also 1 2 2
2 n 1 d1
n1 d 2
2 1
n 2 d1
where 1 = surface tension of water at room temperature.
Substituting the values of 1, n1, n2, d1 and d2 , the surface tension of the given liquid at
room temperature thus becomes known.
Result: -
Discussion: -
Precautions: -
a. The stalagmometer and specific gravity bottle or pyknometer should be
cleaned properly and dried before use.
b. Fit the stalagmometer vertically.
c. The rate of the fall of the drops should be adjusted in a way of having
interval of at least 2 – 3 seconds in successive drops. The number of drops
per minute must be in between 15 – 20.
d. The drops should fall from the tip of the stalagmometer under their own
weight rather than pushing them by force.
Updated on 1st January 2021 by Dr. Kaushik Nath Page 33
Department of Chemical Engineering
e. Wash and dry the stalagmometer after use.
Exercise: -
1. How do you define the term surface tension?
2. What method do you use in laboratory for determination of surface tension?
Briefly explain that method.
3. What formula is used for the calculation of surface tension?
4. How do you measure the density of the liquid?
5. What is the shape of a liquid drop falling from a capillary?
Updated on 1st January 2021 by Dr. Kaushik Nath Page 34
Department of Chemical Engineering
Experiment No. 10
Methods for testing Tar and Bitumen determination of softening point
(IP-198and IS 1208 specifications)
Aim: -
To determine the softening point of a given bituminous material
Apparatus and Chemicals: -
1. 1000 mi of Beaker with cover
2. Thermometer
3. Heating mental
4. Asphaitic material and water
Theory: -
This standard covers the method for the determination of softening point of asphaltic
bitumen & fluxed native asphalt road tar coal tar pitch and blown type bitumen. The
temperature at which the substance attains a particular degree of softening under
specified condition of test.
Procedure: -
Preparation of test sample: -Heat the material to a temperature between 75 0C &
100 0C above its softening point stir until It is completely fluid and free from air
bubbles and water, and filter, if necessary through IS sieve 30(see IS: 460-1953).
Place the rings, previously heated to a temperature approximating to that of
molten material, on a metal plate, which has been coated with mercury and filled
with sufficient melt to give excess above the level of the ring when cooled. After
cooling for 30mins in air, level the material in the ring by removing the excess
with warmed sharp knife.
For material of softening point below 80 0C: -Assemble the apparatus with the
rings, thermometer and ball guides in position; and fill the bath to a height of
50mm. Above the upper surface of the ring with freshly boiled distilled water at a
temperature of 50 0C for 15mins after which place a ball previously cooled to a
temperature of 50C by mean of forceps in each ball guide. Apply heat to the bath
and stir the liquid so that the temperature rises at uniform rate of 50+ 0.50 0C per
minutes until the material softens and allows the ball to pass through the ring. The
Updated on 1st January 2021 by Dr. Kaushik Nath Page 35
Department of Chemical Engineering
rate of temperature rise shall not be averaged over the period of test, & any test in
which the rate of temp rise does not fall within the specified limits after the first 3
mins shall be rejected. Make the determination in duplicate.
For materials of softening above 800 0C: -The procedure for materials of
softening point above 800 0C is similar to that described under 3.2 with the
difference that glycerin is used in place of water in the bath and the starting temp.
of the test is 350 0C. Make the determination in duplicate.
Report:
a. Record, for each ring and ball, the temp, shown by the thermometer at the instant
the sample surrounding the ball touches the bottom plate of the support. If any, or
the bottom of the bath.
b. Report to the nearest 0.50 0C the mean of the temp recorded in duplicate
determination, without correction for the emergent stem of the thermometer, as
the softening point.
Observation table:
Softening point Softening point Softening point Average
Sample in 0C in 0C in 0C Softening point
1 2 3 in 0C
Result: -
Industrial Application: -
Exercise: -
1. What is the difference between asphalt, bitumen and asphaltenes? How are they
produced?
2. Write the significance of softening point.
3. What are the uses of bitumen?
4. What is dewaxing? Name the solvent used for dewaxing.
Updated on 1st January 2021 by Dr. Kaushik Nath Page 36
Department of Chemical Engineering
Experiment No: 11
Determination of smoke point of kerosene by smoke point apparatus
Aim: -
To determine the smoke point of a given sample of kerosene
Apparatus: -
1) Standard lamp with wick.
2) Wick height adjuster.
3) Linear scale to denote the flame height.
Principle: -
Smoke point is an indication of clean burning quality of kerosene. Illumination depends
upon the flame dimensions although it is not related to flame height. Smoke point is
defined as the maximum height of flame in millimeters at which the given oil will burn
without giving smoke. Thus smoke point test enables the burning quality to be measured
by adjusting the wick height to give proper non-smoky flame. Different flame heights are
obtained due to the presence of the different components such as paraffin, naphthenes,
and aromatics. Mainly aromatics contribute smoke; hence removal of aromatics increases
smoke point.
Smoke Point Lamp: -
Updated on 1st January 2021 by Dr. Kaushik Nath Page 37
Department of Chemical Engineering
Specifications: -
Conforms to ASTM D1322; ISO 3014; IP 57; DIN 51406; FTM 791-2107
Included Accessories Cotton Wicks, non-extracted (6)
Dimensions (Dia x H) 7 x 18.5 in. (18 x 47 in.)
Net Weight 10 lbs (4.5kg)
Shipping Weight 16 Ibs (7.3kg)
Shipping Dimensions 5 Cu. ft.
Procedure: -
Take out the lamp & wick from the stand.
Fill the container with given sample of kerosene.
Fix the container into the jacket & light up the wick.
Observe the formation of black smoke from the top.
Observe the color and luminosity of the flame.
Note down the initial height.
Now adjust the wick height slowly by rotating the adjuster & wait for few minutes
to observe the formation of smoke.
Note down the height in millimeter.
Adjust the flame to a non-smoky & luminous condition. Measure the height
which corresponds to actual smoke point of kerosene
Observation Table:-
Flame height Smoke characteristics Luminosity
(In mm.) (Whether smoky / non smoky) (Luminous / non-luminous)
Result: -
Significance and Use: -
This test method provides an indication of the relative smoke producing properties of
kerosene and aviation turbine fuels in a diffusion flame. The smoke point is related to the
Updated on 1st January 2021 by Dr. Kaushik Nath Page 38
Department of Chemical Engineering
hydrocarbon type composition of such fuels. Generally the more aromatic the fuel the
smokier the flame. A high smoke point indicates a fuel of low smoke producing
tendency.
The smoke point (and Luminometer number with which it can be correlated) is
quantitatively related to the potential radiant heat transfer from the combustion products
of the fuel. Because radiant heat transfer exerts a strong influence on the metal
temperature of combustor liners and other hot section parts of gas turbines, the smoke
point provides a basis for correlation of fuel characteristics with the life of these
components.
Exercise: -
1. State the standard tests prescribed for kerosene
2. Define Smoke point and name the processes, which are used to improve smoke
point.
3. What do you meant by ignition temperature of the fuel?
4. What is the difference between lube oil, lubricating oil &gas oil?
Updated on 1st January 2021 by Dr. Kaushik Nath Page 39
Department of Chemical Engineering
Experiment No: 12
Determination of Viscosity by Oswald Viscometer
Aim: -
To determine the relative viscosity of a given liquid with respect to water at different
temperature using Oswald‟s viscometer
Apparatus:
Oswald‟s Viscometer, thermometer, thermostat, pipette, Sp. Gr. Bottle, weight box and a
stop watch.
The apparatus consists of a U-tube with a glass bulb A, made from a thick-walled
capillary glass tube. The two limbs of the U-tube are bent at right angles and end in
narrow bores. One of the ends is shorter and thicker, and the other narrower and longer.
Two glass caps to prevent loss due to evaporation close the ends. The pyknometer is
weighed by suspending it with a wore from the hook of the balance.
Theory:
Viscosity of a solution varies with its concentration. If the viscosities are plotted against
the corresponding concentration, a curve (depending on the nature of the solution) is
obtained by determining the viscosity of the unknown solution, its concentration may be
found out from the curve.
Consider a column of liquid, in a capillary glass tube, flowing downward due to a
pressure difference P. The liquid column in the tube may be considered as being
composed of a large number of concentric cylindrical layers which slides over one
another and moves downward due to the pressure difference. As the liquid layer in
contact with the glass wall tends to move, a shearing effect is produced by the glass wall
and destroys the relative motion of the moving layer. Similarly, each of the moving liquid
layer experiences a shearing effect by its preceding liquid layer. Thus the velocity of the
different liquid layers are not the same, but change uniformly according to their distance
from the glass wall, and its maximum at the center and minimum at the surface of the
wall. The shearing effect or the internal friction which is produced by the glass wall and
the different liquid layers, when a liquid flows, and which opposes the flow of the liquid
is called „viscosity‟ of the liquid. Newton supposed that the force which opposes the
Updated on 1st January 2021 by Dr. Kaushik Nath Page 40
Department of Chemical Engineering
movement of a particular cylindrical liquid layer is proportional to the velocity gradient
du
and to area A of the liquid layer. Thus:
dx
du du
F A or F A --------------------------- (1)
dx dx
When is the co-efficient of viscosity, which is constant for a particular substance at a
du
definite temperature and pressure? When = 1 and A = 1, equation (1) reduces to F =
dx
. Thus from this, coefficient of viscosity may be defined as the force per unit area in
dynes / cm 2 required to maintain a difference of viscosity of 1 cm between two parallel
liquid layers flowing at a distance of 1 cm. The unit of co-efficient of viscosity is called 1
poise.
When the driving force (i.e., the force due to the pressure difference (P) becomes equal to
the viscous-drag, the liquid flows steadily.
For the determination of co-efficient of viscosity of a liquid by Oswald‟s Viscometer the
following equation of Poisculle is employed
P x4 t
------------------------------------- (2)
8lV
When is the co-efficient of viscosity of a liquid which flows uniformly through a
capillary tube of radius x cm and length l cm at the rate of V ml of the liquid in t seconds
and P represents the driving pressure in dynes / cm 2 .
In the Oswald‟s Viscometer the relative viscosity of a liquid is measured. So if t1 and t 2
represent the times required for the same volume of two different liquids to flow through
the same capillary tube, we have for the two liquids
P x 4 t1
1 --------------------------------- (3)
8lV
and
P x 4 t2
2 ------------------------------------- (4)
8lV
Thus dividing eqn. (3) by (4) we get the ratio of the viscosity co-efficient of the two
liquids:
1 p t
1 1 ----------------------------------------- (5)
2 p2 t 2
Since p1 and p2 are proportional to 1 and 2 , the densities of the two liquids
[because p1 h 1 g and p2 h 2 g ] the eqn. (5) becomes
Updated on 1st January 2021 by Dr. Kaushik Nath Page 41
Department of Chemical Engineering
1 1 t1
----------------------------------------- (6)
2 2 t2
Where 2 , 1 , 2 , t1 and t 2 are known 1 can be calculated from eqn. (6).
Procedure:
Clean the Oswald‟s Viscometer thoroughly with chromic acid and water. Drop from a
measuring pipette a definite volume of water into the viscometer through the right hand
limb, D. Maintain a thermostat at constant temperature and note the temperature.
Immerse the viscometer into the thermostat and keep it for sometime, so that it can attain
the constant temperature of the bath. Suck the water with the rubber tube up to a level
above the mark „a‟. Allow the water to flow down the tube. Start the stopwatch when the
level of water is at „a‟ and stop it when the level falls at „b‟. Note the time of fall and
repeat the experiment at least five times. [Difference of time must not exceed 0.2 to 0.3
seconds.]
Dry the viscometer by passing hot air and repeat the experiment at least three times with
the same volume of liquid, whose viscosity is to be determined.
Determine the density of the liquid by a specific gravity bottle in the usual way.
Observations: -
1. Room temperature: --------------
2. Time of flow between two marks: --------------
3. Density of liquid (d1) and of water (d2): ---------------
(Using Specific gravity bottle)
Observation Table: -
Sr Temperature Water Liquid under Experiment
No. in C Time of Time of Mean Time of Time of Mean
flow - 1 flow - 2 time flow - 1 flow - 2 time
1
2
3
4
Updated on 1st January 2021 by Dr. Kaushik Nath Page 42
Department of Chemical Engineering
Calculations:
1. Density of liquid (d1) / Density of water (d2) = Weight of liquid / Weight of
water
2. Relative viscosity of liquid = n1 / n2 = d1t1 / d2t2
Result: -
Discussion: -
Industrial Applications: -
Precautions:
1. The viscometer should be thoroughly cleared (grease free).
2. The viscometer should be held exactly vertical during the experiment.
3. Volume of water and the experimental liquid taken in the viscometer should be
equal.
4. The temperature should be maintained constant during the experiment with the
help of a thermostat. If a thermostat be not available, the temperature at the
beginning as well as at the end of the experiment should be noted carefully, and if
there be any difference, the mean should be recorded as the room temperature.
5. If the experimental liquid be volatile, a pyknometer, instead of a Sp. Gr. Bottle
should be used for determination of density.
Exercise: -
1. What is the principle of Ostwald viscometer?
2. What is viscosity? What are the different units of viscosity?
3. What is the effect of temperature on viscosity?
4. What are the different types of viscometers? Explain their principle.
5. What is the importance of determination of viscosity?
6. What are the viscoelastic fluids? What are their applications?
Updated on 1st January 2021 by Dr. Kaushik Nath Page 43
Department of Chemical Engineering
Experiment No: 13
Determination of Chemical oxygen demand (COD)
Aim: To determine the COD value of a given sample of wastewater.
Apparatus and reagents: -
(1) Standard potassium dichromate solution, 0.04167 M. 12.259 g of K2Cr2O7 was
dissolved in distilled water and diluted to 1000 ml. This reagent undergoes a
six-electron reduction reaction; the equivalent concentration is 0.2500 N.
(2) Ferroin indicator solution: 1.485 g/1, 10 phenanthroline monohydrate and 695
mg FeSO4. 7H2O in distilled water and was diluted to 100 ml.
(3) Standard ferrous ammonium sulfate (FAS) titrant, approximately 0.25 M: 98 g
of Fe (NH4) 2(SO4)2.6H2O was dissolved in distilled water. 20 ml of
concentrated H2SO4 was added to it. The mixture was cooled and diluted to
1000 ml. This solution was standardized against standard K2Cr2O7 solution as
follows:
25 ml of standard K2Cr2O7 was diluted to about 100 ml. 30 ml of concentrated
H2SO4 was added to it and was cooled. It was titrated against FAS titrant using 2-
3 drops ferroin indicator.
Principle:
COD (Chemical oxygen demand) is a measure of any kind of oxidizable impurities
present in the sewage. COD is a measure of both the biologically oxidizable and
biologically inert organic matter present in the sewage sample. It is an important and
quickly measured parameter for stream and industrial waste water analysis and water
treatment plants. A boiling mixture of chromic and sulfuric acids oxidizes most types of
organic matter. A sample is refluxed in strongly acid solution with a known excess of
potassium dichromate (K2Cr2O7). After digestion, the remaining unreduced dichromate is
titrated with ferrous ammonium sulfate to determine the amount of K2Cr2O7 consumed
and the oxidizable matter is calculated in terms of oxygen equivalent.
Procedure: -
For samples with a COD of > 900 mg O2/l, a smaller portion was diluted to 50 ml. 1 g of
Hg SO4 and a few glass beads were added. Then 5 ml of H2SO4 reagent was added very
slowly. The mixture was cooled in ice bath to avoid possible loss of volatile materials. 25
ml of standard K2Cr2O7 solution was added and mixed. The flask was attached to the
condenser and the cooling water was turned on. Remaining sulfuric acid (70 ml) was also
added slowly into the flask with continued swirling. Reflux was carried out for 2 hours. It
was cooled and the condenser was washed down with distilled water. The reflux
condenser was disconnected and the mixture was diluted to about twice its volume with
distilled water. It was cooled down to room temperature and the excess K2Cr2O7 was
Updated on 1st January 2021 by Dr. Kaushik Nath Page 44
Department of Chemical Engineering
titrated against standard FAS, using 2-3 drops of ferroin indicator. The first sharp color
change fro blue-green to reddish-brown was noted which persisted for more than one
minute. In the same manner a blank containing the reagents and a volume of distilled
water equal to that of the sample was also refluxed and titrated.
(A B) M 8000
COD as mg O2/l =
ml of sample
Where
A = ml of FAS used for blank
B= ml of FAS used for sample
M = molarity of FAS and 8000 = milli equivalent weight of oxygen × 1000 ml/l
Precautions: -
1. Adding of suphuric acid reagent to reflux flask should be done slowly and by
shaking the flask. As it is an exothermic reaction the flask should be cooled
during mixing.
2. Smaller volume of sample should be taken in the flask, if it has high COD.
3. End point of the titration should be carefully observed.
Commercial Application: -
COD (Chemical oxygen demand) is one of the most important characteristic parameters
of wastewater. For any wastewater treatment process, the knowledge of COD helps in
determining the amenability of the waste water towards its treatment process.
Exercise: -
1. What is chemical oxygen demand?
2. What do you meant by BOD?
3. Name the impurities, which may interfere in COD determination.
4. Correlate BOD with COD.
5. Discuss the significance of COD determination.
6. How do you define the pollution strength of waste water and industrial waste?
Updated on 1st January 2021 by Dr. Kaushik Nath Page 45
You might also like
- Acropolis Institute of Technology & Research, Indore: BT101 Engg ChemistryNo ratings yetAcropolis Institute of Technology & Research, Indore: BT101 Engg Chemistry7 pages
- Experiment 1: Specific Gravity Determination of Liquids: Physical Pharmacy Laboratory: PrelimNo ratings yetExperiment 1: Specific Gravity Determination of Liquids: Physical Pharmacy Laboratory: Prelim15 pages
- Indian Standard: Methods of Test For Petroleum and Its ProductsNo ratings yetIndian Standard: Methods of Test For Petroleum and Its Products8 pages
- Lecture 3: Petroleum Refining Overview: 3.1 Crude Oil100% (1)Lecture 3: Petroleum Refining Overview: 3.1 Crude Oil2 pages
- To Determine The Melting Point of Organic Compounds Like Naphthalene and Benzoic AcidNo ratings yetTo Determine The Melting Point of Organic Compounds Like Naphthalene and Benzoic Acid7 pages
- Experiment No:1: Determination of Reid Vapour Pressure AimNo ratings yetExperiment No:1: Determination of Reid Vapour Pressure Aim26 pages
- Standard Test Method For Carbon Residue: University of Zakho School of Engineering Petroleum Eng. DepNo ratings yetStandard Test Method For Carbon Residue: University of Zakho School of Engineering Petroleum Eng. Dep5 pages
- Toluene Toluene Toluene Hydrogen Chromium PlatinumNo ratings yetToluene Toluene Toluene Hydrogen Chromium Platinum6 pages
- 2210 - Melting Points and Mixed Melting Points0% (1)2210 - Melting Points and Mixed Melting Points13 pages
- Design Project "Glycerol": Srinivas Reddy Cherukula (09bch020) Sanath Kumar Vellanki (09bch066)100% (1)Design Project "Glycerol": Srinivas Reddy Cherukula (09bch020) Sanath Kumar Vellanki (09bch066)38 pages
- High Density Polyethylene and Low Density PolyethyleneNo ratings yetHigh Density Polyethylene and Low Density Polyethylene10 pages
- Aniline Point Test Apparatus: Instruction Manual FORNo ratings yetAniline Point Test Apparatus: Instruction Manual FOR4 pages
- University of Kirkuk College of Pharmacy: Partition CoefficientNo ratings yetUniversity of Kirkuk College of Pharmacy: Partition Coefficient4 pages
- Furfural A Selective Solvent Petroleum Refining: Kemp, JR.No ratings yetFurfural A Selective Solvent Petroleum Refining: Kemp, JR.8 pages
- Petroleum Refinery Lab. Kinematic Viscosity100% (1)Petroleum Refinery Lab. Kinematic Viscosity11 pages
- Presentation Lecture Slides Petroleum Refinery Engineering100% (1)Presentation Lecture Slides Petroleum Refinery Engineering49 pages
- Determination of Aniline Point of Petroleum SamplesNo ratings yetDetermination of Aniline Point of Petroleum Samples4 pages
- Industrial Hygiene: Instructor - Dr. Tamaghna Chakraborti Ph. No. - (0) 9892770980No ratings yetIndustrial Hygiene: Instructor - Dr. Tamaghna Chakraborti Ph. No. - (0) 989277098037 pages
- Azeotropic Mixtures and Their Separation - PPT Outline Seminar 2No ratings yetAzeotropic Mixtures and Their Separation - PPT Outline Seminar 26 pages
- Boley - 1980 - Determination of Foods Synthetic Colours Using HPLCNo ratings yetBoley - 1980 - Determination of Foods Synthetic Colours Using HPLC11 pages
- Kerosene Isosiv Process For Production of Normal Paraffins: Stephen W. SohnNo ratings yetKerosene Isosiv Process For Production of Normal Paraffins: Stephen W. Sohn6 pages
- Evaluation of Crude Oil: Department of Chemical Engineering, National Institute of Technology, Calicut100% (1)Evaluation of Crude Oil: Department of Chemical Engineering, National Institute of Technology, Calicut38 pages
- Preliminary Distillation of Crude Oil 25No ratings yetPreliminary Distillation of Crude Oil 2519 pages
- Petrochemical Industry: Eng/Bassem FathallaNo ratings yetPetrochemical Industry: Eng/Bassem Fathalla45 pages
- Caking Phenomena in Amorphous Food Powders: M. Aguilera, M. DD Valle and Marcus Ka LNo ratings yetCaking Phenomena in Amorphous Food Powders: M. Aguilera, M. DD Valle and Marcus Ka L7 pages
- CPB30503 Petrochemicals & Petroleum Refining Technology Experiment 4: Determination of Reid Vapor Pressure Full Lab Report100% (2)CPB30503 Petrochemicals & Petroleum Refining Technology Experiment 4: Determination of Reid Vapor Pressure Full Lab Report11 pages
- 3130507-Group Wise Topics For Online PresentationNo ratings yet3130507-Group Wise Topics For Online Presentation2 pages
- Performance and Mechanical Running Tests of Centrifugal Compressors PDF100% (2)Performance and Mechanical Running Tests of Centrifugal Compressors PDF9 pages
- Face Seal Glands: Parker O-Ring HandbookNo ratings yetFace Seal Glands: Parker O-Ring Handbook1 page
- chm150_v5_wk4_debrief_memo_template copyNo ratings yetchm150_v5_wk4_debrief_memo_template copy4 pages
- Osb - Chemical Bonding MCQ With SolutionsNo ratings yetOsb - Chemical Bonding MCQ With Solutions18 pages
- Centrifugal Pump Calculation SpreadsheetNo ratings yetCentrifugal Pump Calculation Spreadsheet8 pages
- Fluid Mechanics and Hydraulic Machines DR R K Bansal PDFNo ratings yetFluid Mechanics and Hydraulic Machines DR R K Bansal PDF287 pages
- Section 5 Equipment Selection, Sizing & Design100% (1)Section 5 Equipment Selection, Sizing & Design43 pages
- Acropolis Institute of Technology & Research, Indore: BT101 Engg ChemistryAcropolis Institute of Technology & Research, Indore: BT101 Engg Chemistry
- Experiment 1: Specific Gravity Determination of Liquids: Physical Pharmacy Laboratory: PrelimExperiment 1: Specific Gravity Determination of Liquids: Physical Pharmacy Laboratory: Prelim
- Indian Standard: Methods of Test For Petroleum and Its ProductsIndian Standard: Methods of Test For Petroleum and Its Products
- Lecture 3: Petroleum Refining Overview: 3.1 Crude OilLecture 3: Petroleum Refining Overview: 3.1 Crude Oil
- To Determine The Melting Point of Organic Compounds Like Naphthalene and Benzoic AcidTo Determine The Melting Point of Organic Compounds Like Naphthalene and Benzoic Acid
- Experiment No:1: Determination of Reid Vapour Pressure AimExperiment No:1: Determination of Reid Vapour Pressure Aim
- Standard Test Method For Carbon Residue: University of Zakho School of Engineering Petroleum Eng. DepStandard Test Method For Carbon Residue: University of Zakho School of Engineering Petroleum Eng. Dep
- Toluene Toluene Toluene Hydrogen Chromium PlatinumToluene Toluene Toluene Hydrogen Chromium Platinum
- Design Project "Glycerol": Srinivas Reddy Cherukula (09bch020) Sanath Kumar Vellanki (09bch066)Design Project "Glycerol": Srinivas Reddy Cherukula (09bch020) Sanath Kumar Vellanki (09bch066)
- High Density Polyethylene and Low Density PolyethyleneHigh Density Polyethylene and Low Density Polyethylene
- Aniline Point Test Apparatus: Instruction Manual FORAniline Point Test Apparatus: Instruction Manual FOR
- University of Kirkuk College of Pharmacy: Partition CoefficientUniversity of Kirkuk College of Pharmacy: Partition Coefficient
- Furfural A Selective Solvent Petroleum Refining: Kemp, JR.Furfural A Selective Solvent Petroleum Refining: Kemp, JR.
- Presentation Lecture Slides Petroleum Refinery EngineeringPresentation Lecture Slides Petroleum Refinery Engineering
- Determination of Aniline Point of Petroleum SamplesDetermination of Aniline Point of Petroleum Samples
- Industrial Hygiene: Instructor - Dr. Tamaghna Chakraborti Ph. No. - (0) 9892770980Industrial Hygiene: Instructor - Dr. Tamaghna Chakraborti Ph. No. - (0) 9892770980
- Azeotropic Mixtures and Their Separation - PPT Outline Seminar 2Azeotropic Mixtures and Their Separation - PPT Outline Seminar 2
- Boley - 1980 - Determination of Foods Synthetic Colours Using HPLCBoley - 1980 - Determination of Foods Synthetic Colours Using HPLC
- Kerosene Isosiv Process For Production of Normal Paraffins: Stephen W. SohnKerosene Isosiv Process For Production of Normal Paraffins: Stephen W. Sohn
- Evaluation of Crude Oil: Department of Chemical Engineering, National Institute of Technology, CalicutEvaluation of Crude Oil: Department of Chemical Engineering, National Institute of Technology, Calicut
- Caking Phenomena in Amorphous Food Powders: M. Aguilera, M. DD Valle and Marcus Ka LCaking Phenomena in Amorphous Food Powders: M. Aguilera, M. DD Valle and Marcus Ka L
- CPB30503 Petrochemicals & Petroleum Refining Technology Experiment 4: Determination of Reid Vapor Pressure Full Lab ReportCPB30503 Petrochemicals & Petroleum Refining Technology Experiment 4: Determination of Reid Vapor Pressure Full Lab Report
- Performance and Mechanical Running Tests of Centrifugal Compressors PDFPerformance and Mechanical Running Tests of Centrifugal Compressors PDF
- Fluid Mechanics and Hydraulic Machines DR R K Bansal PDFFluid Mechanics and Hydraulic Machines DR R K Bansal PDF