0 ratings0% found this document useful (0 votes)
12 viewsAnalysis: Year 12 Practical Homework Exercise 2 Mark Scheme
Analysis: Year 12 Practical Homework Exercise 2 Mark Scheme
Uploaded by
Mariam AhmedThis document is a mark scheme for a Year 12 practical homework exercise analyzing acid-base titrations. It provides the key for 8 marks on analysis of titration calculations and results. It also provides the key for 4 marks on evaluation of the titration technique and accuracy. The mark scheme tests students' ability to perform titration calculations, record results with precision, and evaluate the significance of experimental errors and discrepancies.
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
Analysis: Year 12 Practical Homework Exercise 2 Mark Scheme
Analysis: Year 12 Practical Homework Exercise 2 Mark Scheme
Uploaded by
Mariam Ahmed0 ratings0% found this document useful (0 votes)
12 views2 pagesThis document is a mark scheme for a Year 12 practical homework exercise analyzing acid-base titrations. It provides the key for 8 marks on analysis of titration calculations and results. It also provides the key for 4 marks on evaluation of the titration technique and accuracy. The mark scheme tests students' ability to perform titration calculations, record results with precision, and evaluate the significance of experimental errors and discrepancies.
Original Title
y12_hw_2_ms
Copyright
© © All Rights Reserved
Available Formats
DOC, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
This document is a mark scheme for a Year 12 practical homework exercise analyzing acid-base titrations. It provides the key for 8 marks on analysis of titration calculations and results. It also provides the key for 4 marks on evaluation of the titration technique and accuracy. The mark scheme tests students' ability to perform titration calculations, record results with precision, and evaluate the significance of experimental errors and discrepancies.
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
Download as doc, pdf, or txt
0 ratings0% found this document useful (0 votes)
12 views2 pagesAnalysis: Year 12 Practical Homework Exercise 2 Mark Scheme
Analysis: Year 12 Practical Homework Exercise 2 Mark Scheme
Uploaded by
Mariam AhmedThis document is a mark scheme for a Year 12 practical homework exercise analyzing acid-base titrations. It provides the key for 8 marks on analysis of titration calculations and results. It also provides the key for 4 marks on evaluation of the titration technique and accuracy. The mark scheme tests students' ability to perform titration calculations, record results with precision, and evaluate the significance of experimental errors and discrepancies.
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
Download as doc, pdf, or txt
You are on page 1of 2
YEAR 12 PRACTICAL HOMEWORK EXERCISE 2 MARK SCHEME
Analysis
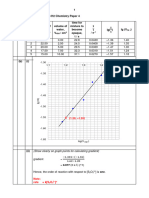
1. HA + NAOH NaA + H2O (1)
2. 27.95 + 28.05 + 28.00
3
= 28 .00 cm3 (1)
3. Moles of alkali = CV = 0.1 x 25 / 1000 = 2.5 x 10-3
1:1 reaction so moles of acid = 2.5 x 10-3
conc = 2.5 x 10-3 /(28/1000)
= 0.0893 mol dm-3 (1)
4. conc. original solution= 0.0893 x 10 = 0.893 mol dm-3
conc. in gdm-3 = 0.893 x 60 = 53.6 gdm-3 (1)
5.
(i) measuring cylinder (0.5/ 25) x 100 = 2.00%
(ii) Volumetric flask (0.5/ 250) x 100 = 0.20%
(iii) pipette (0.05 / 25) x 100 = 0.20%
(iv) burette (0.15 / 28) x 100 = 0.54% (all 4 = 1)
TOTAL = 2.94% (1)
Precision: average titre to 2dp
Both concentrations to 3 sf (1)
Working: clear working
Accurate use of terminology
Correct units (1)
TOTAL 8
Analysing and Evaluation 2001 1
Evaluation
1. First titration poor / probably rough (1)
Three good results/ concordant results
Titration technique good/ results consistent (both = 1)
2. difference = 2.5
= 4.5 % error (both = 1)
3. appreciates discrepancy (4.5%) is greater than total apparatus error (1)
4.
Uses pipette/ burette for measuring supplier’s solution (1)
As apparatus error is smaller (1)
Analysing and Evaluation 2001 2
You might also like
- 2018 H2 Chemistry Paper 4 (Ans)Document8 pages2018 H2 Chemistry Paper 4 (Ans)Justin GohNo ratings yet
- The Binomial TheoremDocument1 pageThe Binomial TheoremMariam Ahmed100% (1)
- Problems 4-25, 4-26, 7-13, 7-14, 7-15 AnswersDocument2 pagesProblems 4-25, 4-26, 7-13, 7-14, 7-15 Answers한공주50% (2)
- Chapter 3 Energy and Momentum Principles - ProblemsDocument3 pagesChapter 3 Energy and Momentum Principles - Problemssoesi thuNo ratings yet
- Student ExperimentDocument15 pagesStudent Experimentkimmy7171No ratings yet
- (Ii) Mustcalculated in (I) (1) A (S) To Na (G) + Gaseous Ions To Solid Nah BecauseDocument67 pages(Ii) Mustcalculated in (I) (1) A (S) To Na (G) + Gaseous Ions To Solid Nah BecauseG M Ali KawsarNo ratings yet
- Lab Report Iodine Clock ReactionDocument6 pagesLab Report Iodine Clock ReactionYoonseo (Elin) ChaNo ratings yet
- Experiment 2 - Viscosity Measurement 2Document5 pagesExperiment 2 - Viscosity Measurement 2Mohammed Al AbdulghaniNo ratings yet
- KiougfhiufghhyDocument65 pagesKiougfhiufghhyG M Ali KawsarNo ratings yet
- Bu Prelim Assign 3 Quiz 1Document1 pageBu Prelim Assign 3 Quiz 1Jhossa EpondulanNo ratings yet
- Chm432 Expt 4Document9 pagesChm432 Expt 4Ievana InsyirahNo ratings yet
- Error Is Mathematical Negative)Document65 pagesError Is Mathematical Negative)G M Ali KawsarNo ratings yet
- 2160 - Mock Exam Sol - Tri 1 22Document6 pages2160 - Mock Exam Sol - Tri 1 22Gaung PradanaNo ratings yet
- (AB) CHEM 1002SEF S102F 2024 Group AssignmentDocument17 pages(AB) CHEM 1002SEF S102F 2024 Group AssignmentsuuktygwNo ratings yet
- Pyramidal: Not Number of Electrons' or Number of Protons in An Element'Document67 pagesPyramidal: Not Number of Electrons' or Number of Protons in An Element'Darly SivanathanNo ratings yet
- M-Caps-03: Physics: (Medical-Classroom Assessment Practice Sheet)Document16 pagesM-Caps-03: Physics: (Medical-Classroom Assessment Practice Sheet)duraibiotechNo ratings yet
- Code-A: Regd. Office: Aakash Tower, 8, Pusa Road, New Delhi-110005 PH.: 011-47623456Document15 pagesCode-A: Regd. Office: Aakash Tower, 8, Pusa Road, New Delhi-110005 PH.: 011-47623456rhevaNo ratings yet
- Correct Cycle in Any Possible Answers 0. 11, 0.110, 0.1111 (CL In) OCIDocument66 pagesCorrect Cycle in Any Possible Answers 0. 11, 0.110, 0.1111 (CL In) OCIG M Ali KawsarNo ratings yet
- UuydghdgdgtertstrwtDocument66 pagesUuydghdgdgtertstrwtG M Ali KawsarNo ratings yet
- Enthalpy/heat Moles AsDocument66 pagesEnthalpy/heat Moles AsG M Ali KawsarNo ratings yet
- KMS CAPEChemu2 02212020 MayJune2021QIG2Document4 pagesKMS CAPEChemu2 02212020 MayJune2021QIG2Donna Cole - CadoganNo ratings yet
- Measurement of Rancidity and Peroxide Value in Vegetable OilDocument7 pagesMeasurement of Rancidity and Peroxide Value in Vegetable OilIgotprime ScammedsNo ratings yet
- MODULE 1 - Dry Lab 1 Activity SheetDocument8 pagesMODULE 1 - Dry Lab 1 Activity SheetCharles Carcel TrinidadNo ratings yet
- N. B. An Energy Diagram Scores 0 MarksDocument65 pagesN. B. An Energy Diagram Scores 0 MarksG M Ali KawsarNo ratings yet
- 2023 HCI P4 Mark SchemeDocument8 pages2023 HCI P4 Mark Schemeanya desilvaNo ratings yet
- Top Q - Units & Dimensions - IVDocument2 pagesTop Q - Units & Dimensions - IVananyanandurkar10No ratings yet
- 9701 Chemistry: MARK SCHEME For The May/June 2009 Question Paper For The Guidance of TeachersDocument8 pages9701 Chemistry: MARK SCHEME For The May/June 2009 Question Paper For The Guidance of TeachersYue ShiNo ratings yet
- Agar Diffusion TestDocument9 pagesAgar Diffusion TestAref DahabrahNo ratings yet
- Physics Fr4 Chapter 1Document16 pagesPhysics Fr4 Chapter 1Bestah Joewellster TeoNo ratings yet
- Data Sheet For Measurements & Evaluation of DataDocument4 pagesData Sheet For Measurements & Evaluation of DataEJ TaylanNo ratings yet
- R3910 Scheme of Valuation/Answer Key: Apj Abdul Kalam Technological UniversityDocument7 pagesR3910 Scheme of Valuation/Answer Key: Apj Abdul Kalam Technological UniversityALAN JOYNo ratings yet
- FIA2 ChemistryDocument8 pagesFIA2 ChemistryBflygraydudeNo ratings yet
- Error PropagationDocument32 pagesError PropagationDora WangNo ratings yet
- Tugas PPM Deny Saputro Arifin 113170039Document9 pagesTugas PPM Deny Saputro Arifin 113170039Vira IrnandaNo ratings yet
- Mark Scheme (Results) January 2008: GCE Chemistry (6246) Paper 1ADocument8 pagesMark Scheme (Results) January 2008: GCE Chemistry (6246) Paper 1Anahian_aziz9050No ratings yet
- CP 4 MSDocument6 pagesCP 4 MSNumpxNump 465No ratings yet
- Sample Applied Statistics and Probability For EngineersDocument7 pagesSample Applied Statistics and Probability For EngineersALBERT DAODANo ratings yet
- Average Values From Many Compounds Used in BondDocument68 pagesAverage Values From Many Compounds Used in BondG M Ali KawsarNo ratings yet
- 2 Ib HL Study Guide Organizer-Measurement & UncertaintyDocument9 pages2 Ib HL Study Guide Organizer-Measurement & UncertaintyMerc 1509No ratings yet
- Pract Memo 2017Document6 pagesPract Memo 2017njabulomadlabane07No ratings yet
- Ch1 - Practice - Questions MSDocument8 pagesCh1 - Practice - Questions MSAnmol SINGHINo ratings yet
- Chemistry 2A - Questions N AnswersDocument6 pagesChemistry 2A - Questions N Answersromanakauki23No ratings yet
- 100 4%6. N. B. Reference To A Single Atom of Chlorine Not Acceptable. (Ii) 0.00258 / Same Number of Moles As Calculated in (I)Document67 pages100 4%6. N. B. Reference To A Single Atom of Chlorine Not Acceptable. (Ii) 0.00258 / Same Number of Moles As Calculated in (I)G M Ali KawsarNo ratings yet
- Solution For Assignment # 2 Sta 5206, 5126 & 4202Document27 pagesSolution For Assignment # 2 Sta 5206, 5126 & 4202Mon LuffyNo ratings yet
- Lab Report - Comparing The Strength of Different Antacids: Data Collection and ProcessingDocument4 pagesLab Report - Comparing The Strength of Different Antacids: Data Collection and ProcessinghavokwreckNo ratings yet
- Mathematical Negative)Document66 pagesMathematical Negative)G M Ali KawsarNo ratings yet
- Boring log - 36 ออกแบบเสาเข็มเจาะ B6229764Document8 pagesBoring log - 36 ออกแบบเสาเข็มเจาะ B6229764Kongsak AkkharawongwhatthanaNo ratings yet
- 9701 Chemistry: MARK SCHEME For The May/June 2014 SeriesDocument5 pages9701 Chemistry: MARK SCHEME For The May/June 2014 SeriesSunny SubashNo ratings yet
- Average Values From Many Compounds Used in BondDocument67 pagesAverage Values From Many Compounds Used in BondG M Ali KawsarNo ratings yet
- Grade 11 MG Term 3 TestDocument10 pagesGrade 11 MG Term 3 Testtebogokulata4No ratings yet
- UncertaintiesDocument17 pagesUncertaintiesChowdhury Mohammed Tawhid TasneefNo ratings yet
- 9701 s09 Ms 31Document7 pages9701 s09 Ms 31Hubbak KhanNo ratings yet
- KC and KP Exam Qu - MSDocument3 pagesKC and KP Exam Qu - MSHannaNo ratings yet
- Exp 7 Spectrophotometry (Memo) - Questions and CalculationsDocument2 pagesExp 7 Spectrophotometry (Memo) - Questions and CalculationsEmeka LouisNo ratings yet
- Solution Endsem Part B 26.11Document6 pagesSolution Endsem Part B 26.11Bhakti KalyankastureNo ratings yet
- N. B. Any Cycle Containing HDocument66 pagesN. B. Any Cycle Containing HG M Ali KawsarNo ratings yet
- Tips For TitrationsDocument4 pagesTips For Titrationsmtayyab zahidNo ratings yet
- Energy SolutionsDocument18 pagesEnergy Solutionsminglei caiNo ratings yet
- Analytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportFrom EverandAnalytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportNo ratings yet
- Advanced Numerical and Semi-Analytical Methods for Differential EquationsFrom EverandAdvanced Numerical and Semi-Analytical Methods for Differential EquationsNo ratings yet
- 12b 1MA1 1H May 2022 SF Mark Scheme (Gold)Document12 pages12b 1MA1 1H May 2022 SF Mark Scheme (Gold)Mariam AhmedNo ratings yet
- MeiaseassDocument1 pageMeiaseassMariam AhmedNo ratings yet
- Y12 To Y12 Organic Chemistry Bridging WorkDocument47 pagesY12 To Y12 Organic Chemistry Bridging WorkMariam AhmedNo ratings yet