The radial gradient of moisture content of silver birch wood in different seasons
Tomczak K., Arkadiusz T., Naskrent B., Jelonek T. (2021). The radial gradient of moisture content of silver birch wood in different seasons. Silva Fennica vol. 55 no. 3 article id 10545. https://doi.org/10.14214/sf.10545
Highlights
- Seasonal variation in moisture content is significant, the greatest moisture content of wood was recorded in winter, and the lowest in summer
- The greatest moisture content on cross-section was observed near to the pith, and lower values near to the bark
- From environmental perspective results of this study may have an impact for log transport planning, weight-scaling systems, lumber drying.
Abstract
Silver birch (Betula pendula Roth) is classified in diffuse-porous wood category. In this case structure of wood tissue is quite similar across whole cross-sectional area. The aim of this study was to analyse cross-section variability of moisture content (MC) of growing silver birch wood, significant hardwood species in Polish forests. Investigations were performed on 120 model trees. In the trunk of each model tree, an increment core was collected at breast height. Samples were collected of 30 different trees in four different seasons. The greatest MC was observed during winter, lowest MC in summer. Differences in MC were statistically significant only between winter versus spring, summer, and autumn. Distribution of MC on cross-section was similar in each season. The greatest average value was observed close to pith, then it was decreasing in bark direction. The greatest difference between observed in spring – 19.51% (p < 0.05) and lowest in autumn – 4.66%. Distribution of green density (GD) on cross section was inverse proportional to MC value. Variations in GD and MC are relevant for log transport planning, weight-scaling systems, lumber drying and dynamic assessment of stiffness. Therefore, from an environmental loss perspective, it is important to determine changes in MC and GD across the year.
Keywords
birch;
Betula pendula;
wood properties;
living trees;
seasonal changes;
trunk cross section
-
Tomczak,
Department of Forest Utilization, Faculty of Forestry and Wood Technology, Poznań University of Life Sciences, Wojska Polskiego 71A, 60-625 Poznań, Poland
 https://orcid.org/0000-0001-5192-0294
E-mail
karol.tomczak@up.poznan.pl
https://orcid.org/0000-0001-5192-0294
E-mail
karol.tomczak@up.poznan.pl

-
Arkadiusz,
Department of Forest Utilization, Faculty of Forestry and Wood Technology, Poznań University of Life Sciences, Wojska Polskiego 71A, 60-625 Poznań, Poland
 https://orcid.org/0000-0003-1140-8282
E-mail
arkadiusz.tomczak@up.poznan.pl
https://orcid.org/0000-0003-1140-8282
E-mail
arkadiusz.tomczak@up.poznan.pl
-
Naskrent,
Department of Forest Utilization, Faculty of Forestry and Wood Technology, Poznań University of Life Sciences, Wojska Polskiego 71A, 60-625 Poznań, Poland
 https://orcid.org/0000-0002-0756-4162
E-mail
bartlomiej.naskrent@up.poznan.pl
https://orcid.org/0000-0002-0756-4162
E-mail
bartlomiej.naskrent@up.poznan.pl
-
Jelonek,
Department of Forest Utilization, Faculty of Forestry and Wood Technology, Poznań University of Life Sciences, Wojska Polskiego 71A, 60-625 Poznań, Poland
 https://orcid.org/0000-0001-9558-9951
E-mail
tomasz.jelonek@up.poznan.pl
https://orcid.org/0000-0001-9558-9951
E-mail
tomasz.jelonek@up.poznan.pl
Received 25 March 2021 Accepted 20 May 2021 Published 15 June 2021
Views 31540
Available at https://doi.org/10.14214/sf.10545 | Download PDF

Supplementary Files
1 Introduction
Silver birch (Betula pendula Roth) occurs in boreal areas throughout Europe, and is the most widespread deciduous species in northern Europe. Its climate optimum is located in the humid and cool climate of the Baltic region, where birch creates solid stands. It accounts for the largest proportion of total volume of growing stock in the Baltic countries (28% in Latvia, 22% in Estonia, 17% in Lithuania). In Belarus (24%) silver birch is also widespread tree species (Hynynen et al. 2010). In Poland, it occupies 6% of forest area (Statistical Yearbook of Forestry in Poland 2018). Silver birch is characterized by high productivity and rapid growth. It is a preferred species for the afforestation of abandoned farmlands (Puchniarski and Sobania 2016; Surminski 1979). In good climate conditions, birch can grow to 20–30 meters in height and to 80 cm diameter at breast height (Puchniarski and Sobania 2016). Birch wood contains high-calorie substances which make it easier to burn (Surminski 1979). High-quality birch wood is used in the plywood industry; moreover, wood of small dimensions is used in the cellulose industry and for firewood (Liepins and Rieksts-Riekstins 2013).
Radial variations of wood properties (including density) have been widely studied (Dobrowolska et al. 2020; Heräjärvi 2004; Tomczak et al. 2018). Typical radial patterns of changes in the functional or material properties of wood vary mainly between species (Longuetaud et al. 2017; Lehnebach et al. 2019). However, some radial trends may be strongly modified by changing growth conditions for the same species ( Liepinš and Rieksts-Riekstinš 2013; Möttönen and Luostarinen 2006).
Birch wood is classified as diffuse-porous. In this case, the structure of the wood tissue is quite similar across the whole cross-sectional area. However, the correlation between annual ring width and wood density is negative (Liepinš and Rieksts-Riekstinš 2013; Giagli et al. 2019), similar to coniferous species. Additionally, radial variation of density is also comparable to that of coniferous species. Heräjärvi (2004) examined the variation of basic density within the stem of two species of birch with different vertical locations. In both cases he found that the density increased from the pith to the bark. Similar results are presented in literature (Helinska-Raczkowska 1996; Fedyukov et al. 2020; Jakubowski et al. 2020).
In the tree trunk, the wood around the pith is defined as juvenile. The width of juvenile wood is influenced by many factors, such as site quality, species, tree growth rate, location and other environmental factors (Kocon 1991; Abdel-Gadir and Krahmer 1993; Helińska-Raczkowska and Fabisiak 1995; Bowyer et al. 2003; Csoka et al. 2005; Alteyrac et al. 2006; Pazdrowski et al. 2005; Tomczak et al. 2007; Pazdrowski et al. 2010). Usually it is from several to several dozen annual rings. Juvenile wood is formed under the strong regulatory (auxin) influence of the active living crown, where growth hormones, particularly indole-3-acetic acid (IAA), are synthesized (Savidge 2001). For this reason, juvenile wood is often called crown-formed wood (Paul 1957). In older trees the concentration of IAA in the bottom part of the tree trunk is lower than in the top part, and mature wood is formed (Kucera 2007). Compared with mature wood, juvenile wood has lower holocellulose and alpha cellulose content, higher lignin and hemicellulose content, smaller, shorter tracheids with thinner walls, and larger microfibril angles. It also has lower tangential and higher longitudinal shrinkage, and poorer strength properties (Zobel and Sprague 1998). In silver birch stems, juvenile wood is located from the pith to the 10–15th annual ring (Bonham and Barnett 2001) or to the 25th ring (Dobrowolska et al. 2020). Compared with mature wood, the density of juvenile wood is lower by approximately 50–100 kg m–3 (Fedyukov et al. 2020; Liepiņš and Liepiņš 2017).
Green density is strongly correlated with moisture content (MC). First of all, an increase in the moisture content causes an increase in the green density of the wood. More porous wood with a lower dry density can store more water, and vice versa (Helinska-Raczkowska 1996; Fromm et al. 2001; Dahlen et al. 2020). For this reason, moisture content and thus the green density of wood vary within the tree (from pith to bark and from stem to crown) (Cinotti 1989; Millers 2013; Tomczak et al. 2018). In case of Scots pine (Pinus sylvestris L.) differences of moisture content distribution on cross-section of the trunk between heartwood and sapwood are statistically significant (Millers 2013). In sessile oak (Quercus petraea (Matt.) Liebl.) moisture content is high as it is the case in non-heartwood species, Tomczak et al. (2018) found that in case of sessile oak difference between sapwood and heartwood is similar.
Another interesting phenomenon is the seasonal variation in moisture content. Pallardy (2008) states that the water content decreases during the summer to a minimum just before leaf fall, and then increases during the autumn after leaf fall reduces transpiration. Beedlow et al. (2017) reported that in Douglas fir (Pseudotsuga menziesii (Mirb.) Franco) trees the maximum relative water content occurred in mid-summer, and the minimum in winter. Markstorm and Hann (1957) obtained different results: they found that the moisture content of the outer and inner sapwood for the Douglas fir, Lodgepole pine (Pinus contorta Douglas ex Loudon) and Engelmann spruce (Picea engelmannii Parry ex Engelm.) was highest during the winter. Clark and Gibbs (1957) presented seasonal changes in moisture content for selected diffuse-porous species growing in Eastern Canada. For example, yellow birch (Betula alleghaniensis Britton) and European aspen (Populus tremula L.) attained a maximum water content in spring and a minimum in autumn. Janiczek and Bobrowski (1952) presented similar results for European beech (Fagus sylvatica L.). In this case the maximum moisture content occurred in March, and the minimum in July and August. Additionally, seasonal changes in moisture content depend on geographical factors and within-stem variability. The moisture content of softwoods is subject to smaller variation than that of hardwoods species (Beedlow et al. 2017; Shottafer and Brackley 1982).
The aim of this study was to analyse cross-section variability of the moisture content of growing silver birch wood, a significant hardwood in Polish forests. Research was carried out in four different seasons. We proposed the hypothesis that the seasonal variation in the moisture content would be significant. Additionally, silver birch is a non-heartwood species, and for this reason moisture content variations will be modified by within-stem wood variability (juvenile wood vs. mature wood). The analyses were conducted in a selected area, and the trees were of a specific age; for this reason, alongside moisture content, oven dry density also needed to be determined and characterized.
2 Materials and methods
2.1 Site selection, preparation of model trees and samples labelling
The study was performed in a birch stand located at alder forest site type (I – site index) in north-western Poland (geographical coordinates: 54°01´N, 14°49´E). The age of the stand was 50 years. Diameter of all measured trees at breast height outside bark (DBH) ranged from 16 to 38 cm with average DBH – 25 cm. The average height of the trees was 23 m (www.bdl.lasy.gov.pl; accessed 20 December 2020). A total of 200 model trees were measured, and among these, 120 model trees were chosen by the Draudt method (Grochowski 1973). Diameters of measured trees were attributed to 3 thickness classes, and Draudt coefficient (C) was calculated by using Eq. 1. Draudt coefficient was 0.6. In the next step, the number of trees obtained in each thickness class was multiplied by the calculated factor of 0.6, resulting in the following for each thickness class representing the number of model trees. In the trunk of each model tree, an increment core was collected at breast height using an increment borer (Fig. 1). Samples were collected in four different seasons and phenological states (Table 1). In each period samples were collected from 30 different trees.
![]()
where:
C – Draudt coefficient; N’ – amount of model trees N – amount of all measured trees.
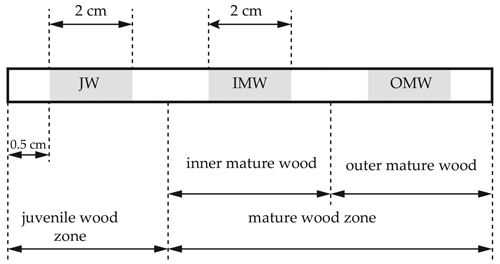
Fig. 1. Increments cores were drilled by Pressler drill at the breast high of silver birch model trees to study radial gradient of moisture content and green density. Increments cores were divided into samples with 2 cm length and 5.15 mm diameter, each was representing a different part of the stem cross-section. The first sample was located 0.5 cm from the pith (1) – juvenile wood (JW). Two other sections represented mature wood. The last section (3) was collected close to the surface of the trunk 0.5 cm from bark – outer mature wood (OMW). Third sample (2) was harvested from the middle of drilling core – inner mature wood (IMW). Between each sample was minimum 1 cm space.
| Table 1. Date of samples collection and phenological state of silver birch trees during each season to study radial gradient of moisture content and green denisty. | ||
| Season | Date | Phenological state |
| Spring | 27th May | Fully expanded leaves |
| Summer | 25th August | End of fruit development |
| Autumn | 3rd November | Dormancy |
| Winter | 7th March | Bud development |
2.2 Measurement of wood properties
After labelling, properties of green wood were measured on site immediately after the increment cores had been collected. In the next step samples were weighed using an electronic scale accurate to 0.01 g. In next step the volume of samples was measured according to dimensional method suggested by Pérez-Harguindeguy et al. (2016). We measured length (L) of each samples using calliper with an accuracy of 0.01 cm. Diameter (D) of each sample was specified according to manufacturer details as 5.15 mm. Based on the measured length and diameter, the volume of each sample was calculated using Eq. 2:
![]()
where:
V – volume; D – diameter of the sample = 5.15 mm; L – length of sample.
After measurements on fresh wood in field, all the samples were transported to the laboratory. In the laboratory, the samples were oven dried at 105 °C. Drying continued until a 0% of water content and constant sample mass was reached. Next, samples were weighted and measured in dried state. Based on all measured properties we specified fallowing wood properties: moisture content (MC), green density (GD) and oven dry density (DD). GD was calculated using Eq. 3, and DD using Eq. 4. Absolute moisture content was calculated using Eq. 5:
![]()
![]()
![]()
where:
mm – mass of fresh sample; ms – mass of dry sample; vf – volume of fresh sample; vd – volume of dry sample.
2.3 Oven-dry density – compared wood samples collected during whole experiment
We observed significant Spearman’s correlation between moisture content and oven-dry density of samples (–0.54). Oven-dry density (volume of dry matter) has an important influence on wood MC – DD regulates how much water the wood can absorb (Tomczak et al. 2018; Pratt et al. 2007). Therefore, in the first step we compared oven-dry density of wood samples collected during whole experiment, to prove that wood samples labelling from 120 different trees in four seasons (30 trees per season) was similar to each other And consequently, DD was not a factor in differentiating the amount of water in the tested wood. Wood samples labelling from living trees in spring, winter and autumn were characterized by similar oven-dry density results. The oven-dry density results of wood samples collected from trees in summer was higher than for other seasons (Table 2). However, the differences between wood samples collected in summer, spring and winter were not statistically significant. We observed significant differences only in case of wood samples from trees labelling in summer and autumn period.
| Table 2. Means of wood oven-dry density collected from silver birch living trees used to specify that wood material of all tress was similar during each period of experiment [g cm–3]. | |||||||||
| Season | Means | N | Std. Dev. | Minimum | Maximum | Q25 | Median | Q75 | |
| Wood samples from | Spring | 0.576 | 90 | 0.078 | 0.389 | 0.759 | 0.512 | 0.576 | 0.637 |
| Summer | 0.606 | 90 | 0.069 | 0.450 | 0.759 | 0.557 | 0.606 | 0.658 | |
| Autumn | 0.575 | 90 | 0.052 | 0.464 | 0.702 | 0.543 | 0.569 | 0.607 | |
| Winter | 0.574 | 90 | 0.067 | 0.397 | 0.706 | 0.522 | 0.594 | 0.626 | |
| Average | 0.583 | 360 | 0.068 | 0.389 | 0.759 | 0.540 | 0.585 | 0.634 | |
2.4 Statistical analyses
In the first step, the Shapiro–Wilk test was performed to verify the normal distribution of data. The test assumes that a statistically significant result permits rejection of the hypothesis of the normal distribution of data. To compare data between samples (independent observations) the non-parametric Kruskal–Wallis test was performed, followed by a Dunn test for multiple comparisons of means from each group of data. Statistical inference was performed at significance level α = 0.05. The program Statistica 13.1 (TIBCO Software Inc., Palo Alto, CA, USA) and the R package (R Core Team, 2020) were used for the calculations.
3 Results
3.1 Moisture content and green density in different seasons
The greatest moisture content was observed during the winter season, and the lowest in summer. In the case of green density, the value was also observed in winter. The lowest green density was recorded in autumn (Table 3). Differences in moisture content were statistically significant only between winter and spring, summer, and autumn. There were no significant differences between the latter three seasons. Moreover we observed significant Spearman’s correlation between moisture and DBH (–0.35) and green density (0.60). In the case of green density there were significant differences between summer and autumn, and also between winter and spring, summer, and autumn. There were no significant differences between spring and autumn or summer (Supplementary file S1: Table A1).
| Table 3. Mean values of moisture content [%] and green density [g m–3] measured from samples collected from silver birch trees in four different seasons to study radial gradient of moisture content and green density. | ||||||||
| Season | Means | N | Std. Dev. | Minimum | Maximum | Q25 | Median | Q75 |
| MC 1 [%] | ||||||||
| Spring | 57.27 | 90 | 21.28 | 16.00 | 152.94 | 47.62 | 53.85 | 60.87 |
| Summer | 54.83 | 90 | 18.15 | 19.35 | 110.00 | 42.31 | 50.00 | 66.67 |
| Autumn | 54.87 | 90 | 13.97 | 26.92 | 95.24 | 45.45 | 54.17 | 65.22 |
| Winter | 81.65 | 90 | 18.45 | 34.62 | 136.84 | 69.23 | 78.71 | 91.30 |
| Average | 62.16 | 360 | 21.32 | 16.00 | 152.94 | 47.91 | 59.09 | 73.38 |
| GD 2 [g cm–3] | ||||||||
| Spring | 0.894 | 90 | 0.090 | 0.681 | 1.125 | 0.829 | 0.899 | 0.957 |
| Summer | 0.931 | 90 | 0.097 | 0.685 | 1.263 | 0.869 | 0.918 | 0.976 |
| Autumn | 0.887 | 90 | 0.079 | 0.673 | 1.098 | 0.845 | 0.880 | 0.934 |
| Winter | 1.032 | 90 | 0.066 | 0.865 | 1.176 | 0.990 | 1.039 | 1.075 |
| Average | 0.936 | 360 | 0.102 | 0.673 | 1.263 | 0.865 | 0.928 | 1.009 |
| 1 MC – moisture content; 2 GD – green density. | ||||||||
3.2 Distribution of examined properties on cross-section
The distribution of moisture content on a cross-section of the trunk was similar in each season. The average value was observed close to the pith; then it decreased in the direction of the bark. The greatest difference between JW and OMW samples (19.51%; p < 0.05) was observed in spring, and the smallest (4.66%) in autumn. As regards the average green density distribution, the value of this feature was greatest close to the bark and decreased toward the pith in each season except autumn. In autumn the lowest green density value was observed in middle samples (inner mature wood). The greatest difference in average GD between juvenile wood and outer mature wood samples (0.104 g cm–3; p < 0.05) was observed in spring, and the lowest (0.008 g cm–3) in autumn (Fig. 2; Fig. 3; Suppl. file S1: Table A2).
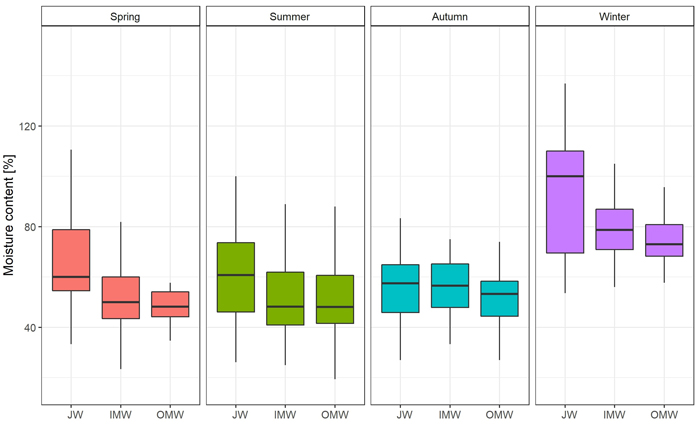
Fig. 2. Moisture content [%] changes on the cross-section (JW – juvenile wood; IMW – inner mature wood; OMW – outer mature wood) of the silver birch trunk by seasons.
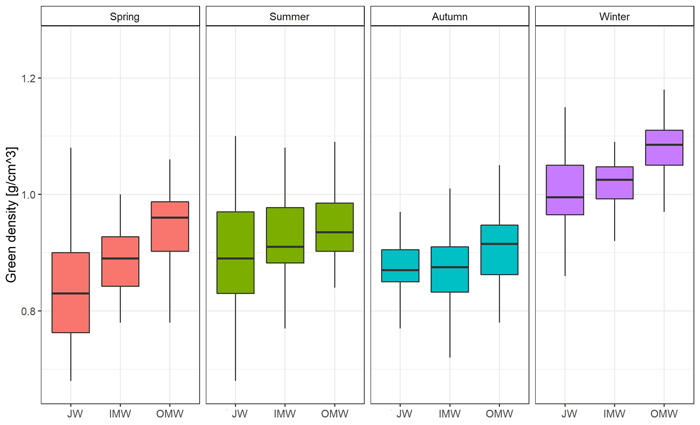
Fig. 3. Green density [g cm–3] changes on the cross-section (JW – juvenile wood; IMW – inner mature wood; OMW – outer mature wood) of the silver birch trunk by seasons.
4 Discussion
Average moisture content in our study was 62%. The lowest MC was observed during summer, although a quite similar result was recorded in autumn; the greatest MC was observed in winter. The difference in moisture content between those seasons was about 27%, confirming that MC depends on season (Janiczek and Bobrowicz 1952; Kubiak and Kosicki 1969; Beedlow et al. 2007; Millers and Magaznieks 2012). During autumn, water in the trunk is transported more slowly, because there is no photosynthesis or transpiration (Kopcewicz et al. 2012). Statistically significant differences were observed between winter and each other season. There were no significant differences between the other seasons. According to Meinzer et al. (Meinzer et al. 2009), the xylem of species with high wood density is less susceptible to cavitation and embolism in dry season.
In the case of MC distribution on a cross-section, the moisture content decreased in a direction from pith to bark in each season. The average MC of samples located near to the pith was about 12.2% greater than that of samples near to the bark. This may be related to the wood density, because high-density wood may accumulate less water than xylem of low density, as was also confirmed by the density results (Pratt et al. 2007; Osunkoya et al. 2007). However, the size of the differences varied between seasons. The greatest differences were observed in spring (19.51%) and winter (17.18%), and the smallest in autumn (4.66%). Statistically significant differences were identified only between the extreme samples (JW and OMW) in spring.
Average green density in our study was approximately 970 kg m–3. This is higher than the values for the GD of birch wood obtained by Wanin (1953) (878 kg m–3) and Wagenführ (1996) (850 kg m–3). Average green density in particular seasons ranged from 919 kg m–3 (spring) to 1069 kg m–3 (winter). Statistically significant differences were identified between autumn and summer and between winter and the other seasons. Similar values of green wood density (no statistically significant differences) were recorded in spring and autumn and in spring and summer.
The pattern of green density distribution was inverse than pattern of moisture content. A similar phenomenon was reported by Helinska-Raczkowska (1996). Variability in the green density distribution on a trunk cross-section in our study was similar in each season. Values of this property increased from the pith to the bark in every season except autumn, when the green density in middle samples was the lowest. These results are similar to those of Dobrowolska et al. (2020), who measured the distribution of GD on a cross-section, and also found that green density increased from pith to bark. Heräjärvi (2004) examined birch trees of a similar age and found that basic density was lowest near to the pith, then increased in the direction of the bark, and then decreased again near to the surface. Möttönen and Luostarinen (2006) compared the properties of silver birch wood from natural forests and plantations in three seasons. They also observed an increase in the basic density of the xylem from pith to surface. The reason for the lower density near to the pith is the occurrence of juvenile wood. Liepiņš and Liepiņš (2017) and Fedyukov et al. (2020) found the density of juvenile wood to be approximately 50–100 kg m–3 lower than that of mature wood. In our study we also observed differences between JW and IMW and OMW. The greatest variation between these wood types was observed in spring, and the smallest in autumn.
The moisture content and density of the wood properties of fresh-felled trees are similar to the properties of wood samples of living trees. Variations are relevant in green density and moisture content for log transport planning, weight-scaling systems, lumber drying and dynamic assessment of stiffness (Chan et al. 2012). Observation and elimination of unnecessary timber transport can reduce the cost of transporting wood (Rix 2014), as well as reducing fuel consumption (Kanzian et al. 2016) and thus GHG (greenhouse gases) emissions and road accidents (Kłapeć et al. 2017). Therefore, from an environmental perspective, it is important to determine the changes in MC and GD across the year.
The experiment was conducted only in one stand to determine the distributions on a cross-section of the trunk in four different seasons of the year. However our results are consistent with previous studies. Therefore, it can be predicted that in an older or younger stand, which will grow on similar or different habitat the results would be comparable. However, this hypothesis needs to be confirmed in further studies, covering not only trees of various ages in various stands, but also from different site type. In each season we measured wood properties from 30 different trees (120 total). The dimension of individual samples were not standardized, moreover samples were characterized by small dimensions. This issue could cause errors in measurements, however this method allowed the exact measurement of moisture and green density at the stem cross-section without felling the trees. Selecting the research material in this way may have resulted in significant differences in wood properties of individual trees between seasons. However, the results of multiple comparison of wood oven dry density collected from all trees during experiment were not statistically significant between wood samples collected in spring, summer or autumn. This means that the studied wood samples drilled from living trees had similar volume of dry matter and therefore oven-dry density did not have significant influence on moisture of examined trees in these seasons. However, the difference between summer and autumn oven-dry density was statistically significant. These results may have an influence on comparisons only between these two seasons; however, during analysis we did not record any significant correlations cases in moisture content. Therefore, it didn’t have a significant impact on the main aim of this study – seasonal differences of wood MC.
5 Conclusions
The aim of this study was to analyse cross-section variability of the moisture content of growing silver birch wood and determine overall variability of MC in growing trees in four different seasons of the year. The average moisture content for the whole year was established at 62.16%. The highest moisture content was recorded in winter, and the lowest in summer. Average green density was 970 kg m–3, with the highest value in winter and the lowest in autumn. However we observed statistically significant differences only in comparison between winter with rest three seasons. These results partially support our hypothesis that the seasonal variation in the moisture content would be significant. Moisture content is strongly correlated with green density, therefore it is important to determine the changes of these properties across the year. The moisture content and density of fresh-felled trees are similar to the properties of wood samples of living trees, therefore from environmental perspective these results may have an impact for log transport planning, weight-scaling systems, lumber drying.
In the case of distribution of moisture content, the lowest MC was observed near to the bark, and higher values near to the pith. The pattern of green density distribution was inverse than pattern of moisture content. The greatest value was observed near to the bark, and the lowest was found closest to the pith. We observed significant differences in spring, winter and summer in the case of green density, and in winter and spring in the case of moisture content. The average difference between samples located juvenile wood zone was greater by app. 12% than in outer mature wood, which was located near to bark. In comparison to heartwood species, especially confers, this situation is exceptional.
Author’s contributions
Karol Tomczak: Data curation and visualization; draft preparation.
Arkadiusz Tomczak: Conceptualization and methodology; draft preparation; supervision.
Bartłomiej Naskrent: Data curation and draft preparation.
Tomasz Jelonek: Draft preparation and supervision.
Openness of research data
Data are available on request from the corresponding author.
Acknowledgments
The authors would like to thank an anonymous reviewer for commenting and improving scientific quality of this paper. The foundation of this work was derived from the Master thesis of Justyna Mikulska.
Funding
This research was co-funded within the framework of the Polish Ministry of Science and Higher Education’s program: “Regional Initiative Excellence” in the years 2019–2022 (No. 005/RID/2018/19).
References
Abdel-Gadir AY, Krahmer RL (1993) Estimating the age of demarcation of juvenile and mature wood in Douglas-fir. Wood Fiber Sci 25: 242–49.
Alteyrac J, Cloutier A, Zhang SY (2006) Characterization of juvenile wood to mature wood transition age in Black spruce (Picea mariana (Mill.) BSP) at different stand densities and sampling heights. Wood Sci Technol 40: 124–138. https://doi.org/10.1007/s00226-005-0047-4.
Beedlow PA, Tingey DT, Waschmann RS, Phillips DL, Johnson MG (2007) Bole water content shows little seasonal variation in century-old douglas-fir trees. Tree Physiol 27: 737–747. https://doi.org/10.1093/treephys/27.5.737.
Beedlow PA, Waschmann RS, Lee EH, Tingey DT (2017) Seasonal patterns of bole water content in old growth Douglas fir (Pseudotsuga menziesii (Mirb.) Franco). Agric For Meteorol 242: 109–119. https://doi.org/10.1016/j.agrformet.2017.04.017.
Benson PH (1957) Juvenile wood in conifers. Report no 2094, Forest Products Laboratory, Forest Service U.S. Department of Agriculture.
Bonham VA, Barnett JR (2001) Fibre length and microfibril angle in silver birch (Betula pendula Roth). Holzforschung 55: 159–162. https://doi.org/10.1515/HF.2001.026.
Bowyer JL, Shmulsky R, Haygreen JG (2003) Forest products and wood science: an introduction 5th edition. Blackwell Publishing, Ames.
Chan JM, Walker JCF, Raymond CA (2012) Variation in green density and moisture content of radiata pine trees in the hume region of New South Wales. Aust For 75: 31–42. https://doi.org/10.1080/00049158.2012.10676383.
Cinotti B (1989) Winter moisture content and frost-crack occurrence in Oak Trees (Quercus petraea Liebl. and Q. robur L.). Ann Des Sci For 46: 614–616. https://doi.org/10.1051/forest:198905ART0138.
Clark J, Gibbs DR (1957) Studies in tree physiology: IV. Further investigations of seasonal changes in moisture content of certain Canadian forest trees. Can J Bot 35: 219–253. https://doi.org/10.1139/b57-021.
Csoka L, Zhu J, Takata K (2005) Application of the fourier analysis to determine the demarcation between juvenile and mature wood. J Wood Sci 51: 309–311. https://doi.org/10.1007/s10086-005-0722-y.
Dahlen J, Schimleck L, Schilling E (2020) Modelling and monitoring of wood moisture content using time-domain reflectometry. Forests 11, article id 479. https://doi.org/10.3390/f11040479.
Dobrowolska E, Wroniszewska P, Jankowska A (2020) Density distribution in wood of European birch (Betula Pendula Roth.). Forests 11, article id 445. https://doi.org/10.3390/f11040445.
Fedyukov VI, Anatolyevna ML, Chernova MS, Tsoy OV, Magalyas NA 2020. A non-destructive prediction method for wood density variations of silver birch trees growing in the middle Volga region, Russia. SEEFOR 11: 85–90. https://doi.org/10.15177/seefor.20-09.
Fromm J, Sautter I, Matthies D, Kremer J, Schumacher P, Ganter C (2001) Xylem water content and wood density in spruce and oak trees detected by high-resolution computed tomography. Plant Physiol 127: 416–425. https://doi.org/10.1104/pp.010194.
Giagli K, Vavrčík H, Fajstavr M, Černỳ J, Novosadová K, Martiník A (2019) Stand factors affecting the wood density of naturally regenerated young silver birch growing at the lower altitude of the Czech Republic region. Wood Res 64: 1011–1022.
Grochowski J (1973) Dendrometria. [Dendrometry]. PWRiL, Warszawa.
Helinska-Raczkowska L (1996) Zmienność wilgotności i gęstości drewna w świeżo ściętych pniach brzozy (Betula Pendula Roth). [Wood moisture content and density variation in the freshly cut birch (Betula pendula Roth)]. Folia For Pol Ser B – Wood Sci 27: 23–30.
Helińska-Raczkowska L, Fabisiak E (1995) Zależność między długością elementów anatomicznych i gęstością drewna brzozy (Betula Pendula Roth). [Relation between the Length of Anatomical Elements and the Density of Birch Wood (Betula Pendula Roth)]. PKTD, PTPN 14: 43–48.
Heräjärvi H (2004) Variation of basic density and Brinell hardness within mature Finnish Betula pendula and B. pubescens stems. Wood Fiber Sci 36: 216–227.
Hynynen J, Niemisto P, Vihera-Aarnio A, Brunner A, Hein S, Velling P (2010) Silviculture of birch (Betula pendula Roth and Betula pubescens Ehrh.) in northern Europe. Forestry 83: 103–119. https://doi.org/10.1093/forestry/cpp035.
Jakubowski M, Tomczak A, Jelonek T, Grzywiński W (2020) Variations of wood properties of birch (Betula pendula Roth) from 23 years old seed orchard. Wood Res 65: 75–86.
Janiczek M, Bobrowicz E (1952) Wilgotność Drewna Świeżego Buków Pomorskich i Karpackich. [Moisture content of fresh wood of Pomeranian and Carpathian European beech trees]. PWRiL, Warszawa.
Kanzian C, Kühmaier M, Erber G (2016) Effects of moisture content on supply costs and CO2 emissions for an optimized energy wood supply network. Croat J For Eng 37: 51–60.
Kłapeć B, Tracz W, Janeczko K (2017) Optymalizacja przewozów drewna nabywanego w jednostkach Lasów Państwowych. [Optimization of the transportation of wood purchased in the State Forests units]. Sylwan 161: 842–850.
Kocon J (1991) Struktura i ultrastruktura drewna młodocianego i dojrzałego jodły pospolitej. [Structure and ultrastructure of juvenile and mature wood of common fir]. Folia For Pol Ser A For 31: 139–150.
Kopcewicz J, Kannenberg K, Szmidt-Jaworska A (2012) Zarys struktury i fizjologii drzew leśnych. [Outline of the structure and physiology of forest trees]. WNUMK, Toruń.
Kubiak M, Kosicki W (1969) Wilgotność drewna drzewostanów sosnowych różnych klas wieku. [Wood moisture of pine stands of different age classes]. PKRiL PTPN 28: 176−183.
Kucera B (2007) A hypothesis relating current annual height increment to juvenile wood formation in Norway spruce. Wood Fiber Sci 26: 152–167.
Lehnebach R, Bossu J, Va S, Morel H, Amusant N, Nicolini E, Beauchêne J (2019) Wood density variations of legume trees in French Guiana along the shade tolerance continuum: heartwood effects on radial patterns and gradients. Forests 10, article id 80. https://doi.org/10.3390/f10020080.
Liepiņš J, Liepiņš K (2017) Mean basic density and its axial variation in Scots pine, Norway spruce and birch steams. Res Rural Dev 1: 21–27. https://doi.org/10.22616/rrd.23.2017.003.
Liepiņš K, Rieksts-Riekstinš J (2013) Stemwood density of juvenile silver birch trees (Betula pendula Roth) from plantations on former farmlands. Balt For 19: 179–186.
Longuetaud F, Mothe F, Santenoise P, Diop N, Dlouhá J, Fournier M, Deleuze C (2017) Patterns of within-stem variations in wood specific gravity and water content for five temperate tree species. Ann For Sci 74, article id 64. https://doi.org/10.1007/s13595-017-0657-7.
Markstrom DC, Hann RA (1957) Seasonal variation in wood permeability and stem moisture content of three Rocky Mountain softwoods. Research Note no. RM-212, USDA Forest Service, Rocky Mountain Forest and Range Experimental Station, Colo.
Meinzer FC, Johnson DM, Lachenbruch B, McCulloh KA, Woodruff DR (2009) Xylem hydraulic safety margins in woody plants: coordination of stomatal control of xylem tension with hydraulic capacitance. Funct Ecol 23: 922–930. https://doi.org/10.1111/j.1365-2435.2009.01577.x.
Millers M (2013) The proportion of heartwood in conifer (Pinus sylvestris L., Picea abies [L.] H. Karst.) trunks and its influence on trunk wood moisture. J For Sci 59: 295–300. https://doi.org/10.17221/29/2013-JFS.
Millers M, Magaznieks J (2012) Scots Pine (Pinus sylvestris L.) stem wood and bark moisture and density influencing factors. In: Annual 18th International Scientific Conference Proceedings, Research for Rural Development, Jelgava, Latvia, 16–18 May 2012, pp 91–97.
Möttönen V, Luostarinen K (2006) Variation in density and shrinkage of birch (Betula pendula Roth) timber from plantations and naturally regenerated forests. For Prod J 56: 34–39.
Osunkoya OO, Sheng TK, Mahmud NA, Damit N (2007) Variation in wood density, wood water content, stem growth and mortality among twenty-seven tree species in a tropical rainforest on Borneo Island. Austral Ecol 32: 191–201. https://doi.org/10.1111/j.1442-9993.2007.01678.x.
Pallardy S (2008) Physiology of woody plants 3rd Edition. Academic Express, Elsevier.
Pazdrowski W, Tomczak A, Kupczyk G, Jelonek T (2005) Juvenile wood volume and proportion vs. selected biometric traits of stems of Scots pine (Pinus sylvestris L.) trees. Acta Soc Bot Pol 74: 269–274. https://doi.org/10.5586/asbp.2005.034.
Pazdrowski W, Borysiak S, Nawrot M, Szymanski M (2010) Stopień krystaliczności celulozy jako wskaźnik dojrzałosci tkanki drzewnej. [The degree of cellulose crystallinity as an indicator of maturity of the wood tissue]. Sylwan 12: 818–827.
Peìrez-Harguindeguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P (2016) New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 61: 167–234. https://doi.org/10.1071/BT12225.
Pratt RB, Jacobsen AL, Ewers FW, Davis SD (2007) Relationships among xylem transport, biomechanics and storage in stems and roots of nine rhamnaceae species of the California Chaparral. New Phytol 174: 787–798. https://doi.org/10.1111/j.1469-8137.2007.02061.x.
Puchniarski TH, Sobania A (2016) Rola brzozy w rekultywacji gruntów. [The role of birch in land recultivation]. WŚ, Warszawa.
Rix JG (2014) Transportation optimization in tactical and operational wood procurement planning. Diss. École Polytechnique de Montréal.
Savidge R (2001) Intrinsic regulation of cambial growth. J Plant Growth Regul 20: 52–77. https://doi.org/10.1007/s003440010002.
Shottafer JE, Brackley AE (1982) An analysis of moisture content variation in eastern spruce and balsam fir in Maine. Technical Bulletin 104, Life Sciences and Agriculture Experiment Station.
Statistical Yearbook of Forestry in Poland 2018.
Surminski J (1979) Właściwości techniczne drewna brzozy i możliwosci jego użytkowania. [Technical properties of birch wood and possibilities of its use]. PWN, Warszawa-Poznań.
Tomczak A, Pazdrowski W, Jelonek T, Stypuła I (2007) Vertical variability of selected macrostructural properties of juvenile wood organization in trunks of Scots pine (Pinus sylvestris L.) trees. Acta Soc Bot Pol 76: 27–33. https://doi.org/10.5586/asbp.2007.003.
Tomczak A, Tomczak K, Smarul N, Rutkowski K, Wenda M, Jelonek T (2018) The gradient of wood moisture within-steam of sessile oak (Quercus petraea (Matt.) Liebl.) in summer. Wood Res 63, article id 12.
Wagenfühe R (1996) Holzatlas. Fachbuchverlag. ISBN 978-3-446-00900-4.
Wanin S (1953) Nauka o Drewnie. [Wood Science]. PWRL, Warszawa.
Zobel BJ, Sprague JR (1998) Juvenile wood in forest trees. Springer Series in Wood Science Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-72126-7.
Total of 55 references.

