Terpenoids PPT. - Terpenes - Natural Products Chemistry
- 1. TERPENOIDS Presentation By: Humna Mehmood BS CHEMISTRY (2017-2021) GPCSF
- 2. NATURAL PRODUCTS Natural products are the compounds which isolate from different natural sources such as plants, animals, microbes, insects, plant pathogens, and endophytes and marine. About 60% of known natural products are terpenoids.
- 3. TERPENOIDS AND TERPENES The terpenoids sometimes called isoprenoids are a large and diverse class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene and the isoprene polymers called terpenes. Terpenes are simple hydrocarbons. Terpenoids are modified class of terpenes with different functional groups and oxidized methyl group moved or removed at various positions. The term ‘terpene’ was originally employed to describe hydrocarbons of the molecular formula C10H16 but now term terpene includes all compounds with formula (C5H8)n. But there is a tendency to call whole group ‘terpenoids’ instead of terpenes and restrict the name terpene to compounds with molecular formula C10H16.
- 4. GENERAL PROPERTIES OF TERPENOIDS Colourless Monoterpenes and sesquiterpenes are volatile Diterpenes and triterpenes are not steam volatile. Tetraterpenes are called carotenoids and are treated as a separate group. Fragrant liquids which are lighter than water Soluble in organic solvent and usually insoluble in water Mostly optically active. Open chain or cyclic unsaturated compounds having one or more double bonds. Undergo addition reaction with hydrogen, halogen, acids, etc. A number of addition products have antiseptic properties. Undergo polymerization and dehydrogenation Easily oxidized nearly by all the oxidizing agents. On thermal decomposition, most of the terpenoids yields isoprene as one of the product.
- 5. IMPORTANCE Used worldwide for the treatment of many diseases. Inhibit different human cancer cells and are used as anticancer drugs such as taxol and its derivatives. Many flavorings and nice fragrances are consisting on terpenes because of its nice aroma. Terpenes and its derivatives are used as antimalarial drugs such as artemisinin and related compounds. A diverse role in the field of foods, drugs, cosmetics, hormones, vitamins, and so on. A role in traditional herbal remedies. Contribute to the scent of eucalyptus the flavors of cinnamon, cloves, and ginger, the yellow color in sunflowers, and the red color in tomatoes. The steroids and sterols in animals are biologically produced from terpenoid precursors.
- 6. ISOPRENE RULE Thermal decomposition of terpenoids give isoprene as one of the product. Otto wallach pointed out that skeleton structure of all naturally occuring terpenoids can be built up of isoprene unit, this is called isoprene rule. Isoprene rule stats that the terpenoid molecules are constructed from two or more isoprene unit.
- 7. SPECIAL ISOPRENE RULE Ingold suggested that ‘the terpenoid molecule are constructed of two or more isoprene units joined in a ‘head to tail’ fashion’. Examples: Open chain monoterpenoids
- 8. SPECIAL ISOPRENE RULE But this rule can only be used as guiding principle and not as a fixed rule. For example carotenoids are joined tail to tail at their central and there are also some terpenoids whose carbon content is not a multiple of five.
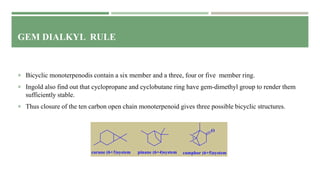
- 9. GEM DIALKYL RULE Ingold pointed that a gem alkyl group affects the stability of terpenoids. He summarized these results in the form of a rule called ‘gem dialkyl rule’ which may be stated as "Gem dialkyl group tends to render the cyclohexane ring unstable where as it stabilizes the three, four and five member rings.” This rule limits the number of possible structure in closing the open chain to ring structure. Thus the monoterpenoid open chain give rise to only one possibility for a monocyclic monoterpenoid i.e the p- cymene structure. All natural monoterpenoids are derivative of p-cymene.
- 10. GEM DIALKYL RULE Bicyclic monoterpenodis contain a six member and a three, four or five member ring. Ingold also find out that cyclopropane and cyclobutane ring have gem-dimethyl group to render them sufficiently stable. Thus closure of the ten carbon open chain monoterpenoid gives three possible bicyclic structures.
- 11. CLASSIFICATION OF TREPENOIDS Terpenoids are divided depending on its carbon units (isoprene units) as shown below.
- 12. 1. MONOTERPENES Monoterpenes consist of 10 carbon atoms with two isoprene units and molecular formula C10H16. Naturally present in the essential and fixed oils of plants and related sources. The compounds belong to this class usually have strong aroma and odor and are used in many pharmaceutical companies. Mixture of different monoterpene-based oils is used as fragrances for making perfumes and in other cosmetics. Most of the monoterpenes are active biologically with strong antibacterial activities. Several studies have shown in vitro and in vivo antitumor activity of many essential oils obtained from plants. The antitumor activity of essential oils of many species has been related to the presence of monoterpenes in their composition.
- 13. CLASSIFICATION Monoterpenes are structurally divided into the three type of compound on the basis of no. of rings present in them. Acyclic monoterpenes Monocyclic monoterpenes Bicyclic monoterpenes
- 14. ACYLIC MONOTERPENES Myrcene (C10H16) It is liquid B.P. 166-168 °C. It is an acyclic monoterpene hydrocarbon which occurs in verbena and bay oils. Ocimene (C10H16) B.P. 81°C/30 mm. Bay Verbena
- 15. ACYLIC MONOTERPENES Citral (C10H16O) This is the important member of the acyclic monoterpenes because stuctures of most of other compounds of this group are based on that of citral. Citral is widely distributed and occurs to an extent of 60-80 percent in lemon grass oil. Citral is a liquid which has smell of lemons. Two geometrical isomers are possible: Trans form Citral-a known as geranial B.P. 118-119°/20mm Cis form Citral-b known as neral B.P. 117- Lemon grass plant
- 16. ACYLIC MONOTERPENES Geraniol and Nerol (C10H18O) Boiling point of geraniol is 229-230 °C/757mm. This is found in many essential oils particularly in rose oil. Reduction of citral produces geraniol but at the same time some nerol is also produced. Nerol is cis form while geraniol is trans form. Nerol is found in various essential oils such as neroli, bergamot etc. Its boiling point is 225-226 °C. ROSE OIL NEROLI PLANT BERGAMOT PLANT AND OIL
- 17. ACYLIC MONOTERPENES Linalool (C10H18O) B.P. 198-199 °C This is optically active compound; (-)-form occurs in rose oil and (+)-form in orange oil. LAVENDER
- 18. ACYLIC MONOTERPENES Citronellal (C10H18O) This is an optically active compound which is found in citronella oil. Citronella Plant
- 19. ACYLIC MONOTERPENES Citronellol and Rhodinol (-)-citronellol occurs in rose and geranium oils and is mixture of the two forms: The (+)-form of citronellol is made commercially by reduction of citronellal with sodium or aluminium amalgam, it also occurs in Java citronella oil. Rhodinol is identical with citronellol but proportion of the two form are diffferent from those which occurs in citronelllol. Java citronella oil Java citronella plant
- 20. MONOCYLIC MONOTERPENES Alpha-terpineol is solid, m.p. is 35° C. It is an aptically active monoterpene occurs in (-)-form,(+)-form and (±) forms. Two other terpineols β-terpineol and γ-terpineol are also known.
- 21. ACYLIC MONOTERPENES Carvone C10H14O B.P. 230 °C/755mm.This is found in various essential oils e.g. spearmint and caraway oils, in optically active forms and also as the recemic modification. Spearmint plant Caraway
- 22. ACYLIC MONOTERPENES Limonene C10H16 B.P. 175.5-176.5 °C This a optically active compound, the (+)-form is present in lemon and orange oils and the (-)-form is present in peppermint oil and the (±) form in turpentine oil. Orange and lemon Turpentine plant
- 23. ACYLIC MONOTERPENES 1 : 8-Cineole (C10H18O) B.P. is 174.4 °C. This occurs in Eucalptua oils. Ascaridole (C10H16O2) B.P. 96-97°/8 mm. Cineoles are oxides and Ascaridole is peroxide. It occurs natually in chenpodium oil. When heated to 130-150° C decomposes with explosive violence. Chenpodium oil Eucalptus oil
- 24. ACYLIC MONOTERPENES Menthol and Menthone Menthol M.P. 34° C, is an optically active compound but only the (-)-form occurs naturally in peppermint oils. Menthone C10H18O B.P. 204° /750 mm. (-)-Menthone occurs in peppermint oil. Menthol Natural menthone
- 25. ACYLIC MONOTERPENES (±)- Pulegone (C10H16O) B.P. 221-222 °C. This occurs in pennyroyal oils. On reduction it gives menthone and on further reduction it give menthol. (-)-Piperitone (C10H16O) B.P. 232-233 °C/768 mm. This occurs in eucalyptus oils and is valuable source of menthone and thymol.
- 26. BICYCLIC MONOTERPENES Bicyclic monoterpenes can be divided into three classes according to size of second ring, the first being a six membered ring in each class. Class 1: 6- + 3-membered ring Class 2: 6- + 4-membered ring Class 3: 6- + 5-membered ring It is important to note that the two rings do not lie in one plane but are almost perpendicular to each other.
- 27. BICYCLIC MONOTERPENES Class 1: 6- + 3-membered ring Thujone and its derivatives The members of this group which occurs naturally are the following:
- 28. BICYCLIC MONOTERPENES Carane and its derivatives It appears that only three carane occurs naturally.
- 29. BICYCLIC MONOTERPENES Class 2: 6- + 4-membered ring Pinane is a parent compound of this group. It is a synthetic substance. This exists in two isomeric forms, cis and trans as a pair of enantiomorphs. Alpha pinene is most important compound of this group found in turpentine oil. It is a liquid b.p. 156 °C.
- 30. BICYCLIC MONOTERPENES Class 3: 6- + 5-membered ring Camphane: Camphane C10H18 is solid synthetic compound and may be prepared from camphor. Camphane is an optically inactive compound.
- 31. BICYCLIC MONOTERPENES Camphor occurs in nature in the camphor tree in Formosa and Japan. It is optically active; the (+) and (-) forms occur in nature. It is solid. It is obtained by steam distillation of wood, leaves or bark of camphor tree. It is used in pharmaceutical preparation because of its analgesic. Stimulant for heart muscles. Expectorant and antiseptic properties. It is used in manufacture of celluloid, smokeless powder and explosives. It is also used as moth repellent.
- 32. 2. SESQUITERPENES Sesquiterpenes are the class of secondary metabolites consisting of three isoprene units (C15H24). Sesquiterpenes are also found in the form of lactone ring. Many of the latex in latex-producing plants contain sesquiterpene, and these are potent antimicrobial and anti-insecticidal agent. Artemisinin, a sesquiterpene lactone, one of the most active compounds in Artemisia annua shoots and roots. They are found in linear, cyclic and bicyclic forms.
- 33. 3. DITERPENES Diterpenoids belong to a versatile class of chemical constituents found in different natural sources having C20H32 molecular formula and four isoprene units. This class of compounds showed significant biological activities including anti-inflammatory, antimicrobial, anticancer, and antifungal activities. Some of the diterpenes also have cardiovascular activity, such as grayanotoxin, forskolin, eleganolone, marrubenol, and 14-deoxyandrographolide. Kaurane and pimarane-type diterpenes are also biologically active metabolites isolated from the roots and leaves of different plants.
- 34. 4. SESTERPENES Sesterpenes consist of 25 carbon atoms with 5 isoprene units and molecular formula C25H40. These are naturally present in the fungus, marine organism, insects, sponges, lichens, and protective waxes of insects. These types of compounds are biologically active having anti-inflammatory, anticancer, antimicrobial, and antifungal activities. Sponge ircina felix
- 35. 5. TRITERPENES A major class of secondary metabolites are known as triterpenes and it usually contains 30 carbon atoms consisting of 6 isoprene units. It is derived from the squalene biosynthetic pathway. Triterpenes have many methyl groups and it can be oxidized into alcohols, aldehydes, and carboxylic acids, which make it complex and differentiate it biologically. Triterpenes have many active sites for the glycosylation which converts it into another big class of compounds, namely, saponins (triterpene glycoside).
- 36. 6. POLYTERPENOIDS Polymeric isoprenoid hydrocarbons have also been identified. Rubber is undoubtedly the best known and most widely used compound of this kind. It occurs as a colloidal suspension called latex in a number of plants, ranging from the dandelion to the rubber tree (Hevea brasiliensis). Rubber is a polyene, and exhibits all the expected reactions of the C=C function. Bromine, hydrogen chloride and hydrogen all add with a stoichiometry of one molar equivalent per isoprene unit. Ozonolysis of rubber generates a mixture of levulinic acid and the corresponding aldehyde. Pyrolysis of rubber produces the diene isoprene along with other products.
- 37. 6. POLYTERPENOIDS The double bonds in rubber all have a Z-configuration, which causes this macromolecule to adopt a kinked or coiled conformation. Gutta-percha (structure above) is a naturally occurring E-isomer of rubber. Here the hydrocarbon chains adopt a uniform zig-zag or rod like conformation, which produces a more rigid and tough substance. Uses of gutta-percha include electrical insulation and the covering of golf balls.
- 38. ISOLATION OF MONO AND SESQUITERPENOIDS Both mono and sesquiterpenoids have common source i.e. essential oils. Their isolation is carried out in two steps: 1. Isolation of essential oils from plant parts 2. Separation of Terpenoids from essential oils
- 39. ISOLATION OF MONO AND SESQUITERPENOIDS Isolation of essential oils from plant parts The plants having essential oils generally have the highest concentration at some particular time. Therefore better yield of essential oil plant material have to be collected at this particular time. e.g. From jasmine at sunset. There are four methods of extractions of oils. 1. Expression method 2. Steam distillation method 3. Extraction by means of volatile solvents 4. Adsorption in purified fats Steam distillation is most widely used method.
- 40. ISOLATION OF MONO AND SESQUITERPENOIDS Separation of Terpenoids from essential oils A number of terpenoids are present in essential oil obtained from the extraction. Definite physical and chemical methods can be used for the separation of terpenoids. They are separated by fractional distillation. The terpenoid hydrocarbons distill over first followed by the oxygenated derivatives. More recently different chromatographic techniques have been used both for isolation and separation of terpenoids.
- 41. GENERAL METHODS OF STRUCTURE ELUCIDATION 1. Molecular formula: molecular formula is determined by usual quantitative analysis and mol.wt determination methods and by means of mass spectrometry. If terpenoid is optically active, its specific rotation can be measured. 2. Nature of oxygen atom present: If oxygen is present in terpenoids its functional nature is generally as alcohol aledhyde, ketone or carboxylic groups. Presence of –OH Group: Presence of –OH group can be determined by the formation of acetates with acetic anhydride and benzoyate with 3.5-dinitirobenzoyl chloride. Primary alcoholic group undergo esterification more readily than secondary and tertiary alcohols. Presence of >C=O group If carbonyl function is in the form of aldehyde it gives carboxylic acid on oxidation without loss of any carbon atom whereas the ketone on oxidation yields a mixture of lesser number of carbon atoms.
- 42. GENERAL METHODS OF STRUCTURE ELUCIDATION 3. The presence of olefinic double bond is confirmed by means of bromine, and number of double bond determination by analysis of the bromide or by quantitative hydrogenation or by titration with monoperpthalic acid. Presence of double bond also confirmed by means of catalytic hydrogenation or addition of halogen acids. Number of moles of HX absorbed by one molecule is equal to number of double bonds present. 4. Dehydrogenation give carbone skelton On dehydrogenation with sulphur, selenium, polonium or palladium terpenoids converted to aromatic compounds. Examination of these products the skelton structure and position of side chain in the original terpenoids can be determined.
- 43. GENERAL METHODS OF STRUCTURE ELUCIDATION 5. Oxidative degradation has been the parallel tool for elucidating the structure of terpenoids. Reagents for degradative oxidation are ozone, acid, neutral or alkaline potassium permanganate, chromic acid, sodium hypobromide, osmium tetroxide, nitric acid, lead tetra acetate and peroxy acids. Since oxidizing agents are selective, depending on a particular group to be oxidized, the oxidizing agent is chosen with the help of structure of degradation products 6. Number of the rings present With the help of general formula of corresponding parent saturated hydrocarbon, number of rings present in that molecule can be determined.
- 44. GENERAL METHODS OF STRUCTURE ELUCIDATION 7. Spectroscopic studies All the spectroscopic methods are very helpful for the confirmation of structure of natural terpenoids and also structure of degradation products. The various methods for elucidating the structure of terpenoids are: 1. UV spectroscopy 2. IR spectroscopy 3. NMR spectroscopy 4. Mass spectroscopy 5. X-ray analysis
- 45. GENERAL METHODS OF STRUCTURE ELUCIDATION UV spectroscopy: In terpenes containing conjugated dienes or α,β-unsaturated ketones, UV spectroscopy is very useful tool for detection of the conjugation. IR spectroscopy: useful in detecting functional groups such as hydroxyl group (~3400cm-1) or an oxo group (saturated 1750- 1700cm-1).
- 46. GENERAL METHODS OF STRUCTURE ELUCIDATION NMR spectroscopy : useful to detect and identify double bonds, to determine the nature of end group and also the number of rings present, and also to reveal the orientation of methyl group in the relative Position of double bonds. Mass spectroscopy: widely used as a means of elucidating structure of terpenoids. Used for Determining mol.Wt., Mol. Formula, nature of functional groups present and relative positions of double bonds. X-ray analysis: This is very helpful technique for elucidating structure and Stereochemistry of terpenoids.
- 47. BIOSYNTHESIS OF TERPENOIDS The biosynthesis of all terpenoids from simple, primary metabolites can be divided into four overall steps: Synthesis of the fundamental precursor IPP. Repetitive additions of IPP to form a series of prenyl diphosphate homolog, which serve as the immediate precursors of the different classes of terpenoids. Elaboration of these allylic prenyl diphosphates by specific terpenoid synthases to yield terpenoid skeletons. Secondary enzymatic modifications to the skeletons (largely redox reactions) to give rise to the functional properties and great chemical diversity of this family of natural products.
- 48. BIOSYNTHESIS OF TERPENOIDS Synthesis of IPP Although terpenoid biosynthesis in plants, animals, and microorganisms involves similar classes of enzymes, important differences exist among these processes. In particular, plants produce a much wider variety of terpenoids than do either animals or microbes. difference reflected in the complex organization of plant terpenoid biosynthesis at the tissue, cellular, sub cellular, and genetic levels. The production of large quantities of terpenoid natural products as well as their subsequent accumulation, emission, or secretion is almost always associated with the presence of anatomically highly specialized structures. The glandular trichomes and secretory cavities of leaves and the glandular epiderms of flower petals generate and store or emit terpenoid essential oils that are important because they encourage pollination by insects.
- 49. BIOSYNTHESIS OF TERPENOIDS The organization of terpenoid metabolism exists at the subcellular level. The sesquiterpenes (C15), triterpenes (C30), and polyterpenes appear to be produced in the cytosolic and endoplasmic reticulum (ER) compartments. whereas isoprene, the monoterpenes (C10), diterpenes (C20), tetraterpenes (C40), and certain prenylated quinones originate largely, if not exclusively, in the plastids.
- 50. BIOSYNTHESIS OF TERPENOIDS Acetate/mevalonate pathway Hydroxymethylglutaryl-CoA reductase, an enzyme in the acetate/mevalonate pathway, is highly regulated. The basic enzymology of IPP biosynthesis by way of the acetate/mevalonate pathway is widely accepted This cytosolic IPP pathway involves the two-step condensation of three molecules of acetyl-CoA catalyzed by thiolase and hydroxymethylglutaryl- CoA synthase. The resulting product, 3-hydroxy-3 methylglutaryl- CoA (HMG-CoA), is subsequently reduced by HMG-CoA reductase in two coupled reactions that form mevalonic acid. Two sequential ATP-dependent phosphorylations of mevalonic acid and a subsequent phosphorylation/elimination assisted decarboxylation yield IPP.
- 51. SYNTHEIS OF IPP IN PLASTIDS: In plastid, IPP is synthesized from pyruvate and glyceraldehyde 3-phosphate. In this pathway, pyruvate reacts with thiamine pyrophosphate (TPP) to yield a two-carbon fragment, hydroxyethyl-TPP, which condenses with glyceraldehyde 3- phosphate.TPP is released to form a five-carbon intermediate, 1-deoxy-D-xylulose 5-phosphate, which is rearranged and reduced to form 2-C-methyl-D- erythritol 4-phosphate and subsequently transformed to yield IPP. Discovery of this new pathway for IPP formation in plastids suggests that these organelles, presumed to have originated as prokaryotic endosymbionts, have retained the bacterial machinery for the production of this key intermediate of terpenoid biosynthesis. Feeding studies distinguish two pathways of isoprenoid biosynthesis.
- 52. BIOSYNTHESIS OF SUBCLASSES The major subclasses of terpenoids are biosynthesized from the basic five-carbon unit, IPP, and from the initial prenyl (allylic) diphosphate, dimethylallyl diphosphate, which is formed by isomerization of IPP. In reactions catalyzed by prenyltransferases, monoterpenes (C10), sesquiterpenes (C15), and diterpenes (C20) are derived from the corresponding intermediates by sequential head to- tail addition of C5 units. Triterpenes (C30) are formed from two C15 (farnesyl) units joined head-to-head, and tetraterpenes (C40) are formed from two C20 (geranylgeranyl) units joined head-to-head.
- 53. PRENYLTRANSFERASE AND TERPENE SYNTHASE REACTIONS Prenyltransferase enzymes generate the allylic diphosphate esters Geranyl diphosphate (GPP), Farnesyl diphosphate (FPP), and Geranylgeranyl diphosphate (GGPP). Reactions that these compounds undergo (often cyclizations), which are catalyzed by terpene synthases, yield a wide variety of terpenoid compounds. Repetitive addition of C5 units is carried out by prenyltransferases.
- 54. PRENYLTRANSFERASE AND TERPENE SYNTHASE REACTIONS Isomerization of IPP by IPP isomerase produces the allylic isomer dimethylallyl diphosphate (DMAPP), which is considered the first prenyl diphosphate. The reactive primer DMAPP undergoes condensation with IPP to yield the C10 intermediate GPP. Repetition of the reaction cycle by addition of one or two molecules of IPP provides FPP (C15) or GGPP (C20), respectively. The electrophilic elongation reactions that yield C10, C15, and C20 prenyl diphosphates are catalyzed by enzymes known collectively as prenyltransferases. GPP, FPP, and GGPP are each formed by specific prenyltransferases named for their products (e.g., farnesyl diphosphate synthase).
- 55. THANK YOU