Lab 4
Download as PPT, PDF1 like2,120 views
This document discusses different types of chemical reactions including decomposition, synthesis, combustion, double replacement, and single replacement reactions. It provides examples of each type of reaction and explains the key features that define them. Double replacement reactions are highlighted, where a metal replaces a metal in a compound and a nonmetal replaces a nonmetal, forming a precipitate if one of the products is insoluble. Guidelines are provided for writing molecular, total ionic, and net ionic equations for double replacement reactions.
1 of 35
Downloaded 29 times
















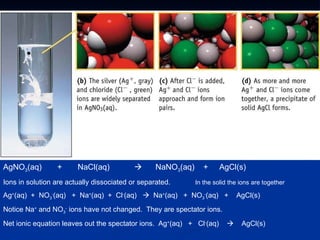

















Recommended
Precipitation reaction



Precipitation reactionbhagwadgeeta 1. The document describes a precipitation reaction experiment to investigate which ionic compounds are soluble and insoluble when different metal and non-metal ion solutions are mixed.
2. Solubility rules are provided to make hypotheses about which reactions will produce precipitates.
3. The experiment involves adding drops of metal ion solutions to drops of non-metal ion solutions in a spotting plate and recording any precipitate formation and color.
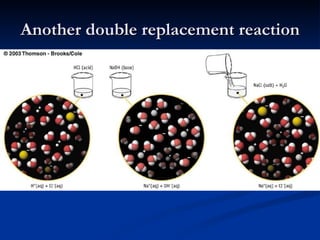
Single & double replacement reactions



Single & double replacement reactionsmartykilroy 1. The document discusses single and double replacement reactions, including the activity series of metals and halogens that can be used to predict products.
2. Single replacement reactions involve one element replacing another in a compound, following the activity series.
3. Double replacement reactions, also called metathesis reactions, involve two compounds exchanging ions when a new substance like water, gas, or precipitate is formed.
Precipitates 



Precipitates johnwest This document discusses precipitation reactions and salt families. It provides the following key points:
1. Salts are produced from chemical reactions between acids and other substances. Each acid produces a characteristic family of salts, such as sulfates from sulfuric acid and nitrates from nitric acid.
2. Precipitation occurs when two solutions are mixed and an insoluble compound forms, coming out of solution as a solid precipitate. Solubility rules can be used to predict whether a precipitation reaction will occur.
3. Practical experiments are described to mix solutions of substances like lead nitrate and barium chloride and observe any precipitates that form based on the solubility rules. Word equations are also written for precipitation
Precipitation reactions



Precipitation reactionsTanya Wood When sodium chloride dissolves in water, the ions separate from each other into positive sodium ions and negative chloride ions. This dissolving process can be represented by the equation NaCl(s) → Na+(aq) + Cl-(aq). The solubility of compounds is based on rules regarding which ions combine to form soluble or insoluble compounds. Compounds that do not dissolve in water and remain as a solid are considered precipitates, and chemical reactions where precipitates form are called precipitation reactions.
chemistry f4 chapter 8 salt



chemistry f4 chapter 8 salta_yu This document provides information about synthesizing and analyzing salts in chemistry. It discusses:
1) How to synthesize salts by reacting acids with metals, metal oxides, metal carbonates. Salts can be soluble or insoluble depending on their constituents.
2) Qualitative analysis of salts including color, gas tests, effects of heat. Salts can be identified by the gas produced or metal oxide left behind when heated.
3) Confirmatory tests to identify cations and anions in unknown salts using sodium hydroxide, ammonium hydroxide, and other reagents. Precipitate observations determine the present ions.
Precipitation react2



Precipitation react2pinillafelisa This document summarizes an experiment on double displacement precipitation reactions. Various metal nitrates and chlorides were reacted and precipitates were observed and recorded. Silver nitrate produced precipitates with potassium iodide (silver iodide, yellow), sodium chloride (silver chloride, white), sodium hydroxide (silver hydroxide, brown), iron(III) chloride (silver chloride, light yellow), sodium carbonate (silver carbonate, light green), and copper(II) sulfate (silver sulfate, very light blue). Lead(II) nitrate also produced precipitates with the same reactants. The conclusion is that silver and lead ions form the most precipitates in these double displacement reactions.
Reactions EOC Review



Reactions EOC Reviewjglanville The document discusses different types of chemical reactions including acid-base reactions where an acid and base react to form a salt and water, precipitation reactions where insoluble compounds form solid precipitates, and oxidation-reduction reactions where electrons are lost or gained. Examples of each type of reaction are provided and rules for determining oxidation states and predicting products are explained.
Other Chem Presentation :))



Other Chem Presentation :))Flora Palabrica The document describes a classic scheme for qualitatively analyzing 21 common cations. It involves a series of preliminary tests including adding HCl, NaOH, NH3, H2S, Na2CO3, and H2SO4. Certain cations form characteristic precipitates that allow their identification. The tests are used to separate the cations into groups, with Group I forming precipitates with HCl, Group II with H2S in acid, and Group III with H2S in base. Flame tests can also help identify some soluble cations.
The Periodic Table



The Periodic TableArrehome 1) The document discusses the periodic table and properties of Group 1 elements (alkali metals).
2) Group 1 elements have one electron in their outer shell and form ions with a +1 charge. Their compounds include oxides, chlorides, sulfates and carbonates.
3) Experiments show the reactivity of Group 1 metals increases down the group with francium being the most reactive and reacting explosively with water. The reactions with water produce alkali metal hydroxides and hydrogen gas.
Revision on acid base and salt = with answers



Revision on acid base and salt = with answersMRSMPC This document provides information about chemistry revision on acids, bases and salts. It discusses soluble and insoluble salts such as chlorides, sulphates and nitrates. It also describes methods for preparing soluble and insoluble salts, including the titration and solid acid methods. The document further discusses the preparation of copper(II) sulphate through the reaction of copper(II) oxide with sulphuric acid, and provides chemical tests to identify the copper and sulphate ions.
Solubility



SolubilityLumen Learning Introduces the concepts involved in predicting whether substances are soluble or insoluble. Precipitation (exchange) reactions are also discussed, along with ionic equations and net ionic equations. General Chemistry
Chapter 8 : SALTS



Chapter 8 : SALTSAndromendas Rizal This document discusses the preparation and classification of salts. Salts are formed through the replacement of hydrogen ions in acids by metal ions or ammonium ions. There are two main methods for preparing salts - neutralization and precipitation. Neutralization involves reacting an acid with a metal, alkali, oxide or carbonate to form a soluble salt. Precipitation involves mixing two aqueous solutions of soluble salts to form an insoluble salt precipitate. The document provides examples of preparing various salts such as potassium chloride and lead chloride. It also discusses classifying salts as soluble or insoluble and purifying soluble salts through recrystallization.
Chapter 8 salt part 1



Chapter 8 salt part 1Syaurah Ashikin The document discusses learning outcomes about salts. It defines a salt as a compound formed when a hydrogen ion from an acid is replaced by a metal ion or ammonium ion. Examples of commonly used salts include NaCl, MSG, and CaCO3. Salts can be soluble or insoluble depending on their cation and anion. The document also describes different methods for preparing soluble and insoluble salts, such as titration, evaporation/heating, cooling/crystallization, filtration, and drying.
Qualitative analysis 1



Qualitative analysis 1Mark Selby The basic chemistry you need to understand in order to start your practical work on Qualitative Analysis.
Chemistry prediction of products and workshop homework



Chemistry prediction of products and workshop homeworkWilliard Joshua Jose This document provides an overview of different types of chemical reactions including:
1) Combination/synthesis reactions where elements or compounds combine to form new substances
2) Decomposition reactions where a single compound breaks down into simpler substances
3) Combustion reactions where a substance reacts with oxygen to form oxides
4) Displacement reactions where an element replaces another in a compound
5) Double displacement reactions where ions are swapped between reactants to form new compounds
It then provides a series of practice problems for students to write balanced chemical equations for different laboratory scenarios involving these reaction types.
Chapter 8 Salt part 6



Chapter 8 Salt part 6Syaurah Ashikin The document describes the process and tests used in qualitative analysis to identify salts based on their physical properties, reaction to heat, and tests to detect specific cations and anions. It provides details on observing the color and solubility of salts, conducting gas tests, and using confirmatory tests to identify ions like Fe2+, Fe3+, Pb2+, and NH4+. The qualitative analysis plan involves examining the salt's physical properties, heating it, testing for cations and anions, and then confirming the identities of ions present.
SPM Form 4 Chapter 8 Salt



SPM Form 4 Chapter 8 Saltyuenkei This document summarizes the solubility of different types of salts in water. It states that hydroxides are generally insoluble except for potassium and sodium hydroxide. Oxides are also largely insoluble except for potassium and sodium oxide. Carbonates are more soluble, with sodium, potassium, and ammonium carbonates all soluble. Sulphates and chlorides are also largely soluble, except for a few exceptions like barium and lead salts. Nitrates and salts of sodium and potassium are all soluble in water. It also provides tests to identify different cations and anions in salts.
3. qualitative analysis



3. qualitative analysisPluviose Qualitative analysis is used to identify the cations and anions present in an unknown chemical substance. Cations such as sodium, calcium, and ammonium can be identified using sodium hydroxide and ammonia solutions. Anions like chloride, nitrate, and sulfate can be identified through chemical tests involving silver nitrate, sodium hydroxide with aluminum foil, and barium chloride solutions respectively. These tests produce characteristic precipitates or gas emissions to reveal the ions present. Dilute nitric acid is first added to remove any interfering carbonate ions.
Preciptation reactions 1



Preciptation reactions 1felisapinilla This document describes an experiment on precipitation reactions involving double displacement reactions. A variety of cation and anion solutions were mixed, including AgNO3, Pb(NO3)2, CaCl2, KI, NaCl, NaOH, FeCl3, KNO3, Na2CO3, and CuSO4. Precipitates formed when AgNO3 was mixed with KI (silver iodide), NaCl (silver chloride), CuSO4 (silver sulfate), Na2CO3 (silver carbonate), and FeCl3 (silver chloride). Precipitates also formed when Pb(NO3)2 was mixed with KI (lead iodide), NaCl (lead chloride), Na2
Transition Metals and Complexes, Vanadium and Chromium metal



Transition Metals and Complexes, Vanadium and Chromium metalSidra Javed Shapes and Color of Coordinations Compounds depends upon the Ligands and d-d transition of electrons in the central elements
Redoxhints



RedoxhintsTimothy Welsh This document summarizes important oxidizers and reducers formed in redox reactions under different conditions. It lists common oxidizing agents like MnO4-, Cr2O7-2, and HNO3 that form reduced products like Mn(II), Cr(III), and NO in acid solutions. It also lists common reducers like halide ions, metals, and sulfite ions that form oxidized products like halogens, metal ions, and SO4-2. The document concludes that redox reactions involve electron transfer between oxidizing and reducing agents, and that acidic or basic conditions often indicate a redox reaction will occur.
Test for cat ions an ions



Test for cat ions an ionsShazeel Dhiffushi This document provides information on common cation and anion tests in chemistry. It lists several cations (aluminum, ammonium, calcium, copper, iron, zinc, lead) and their reactions when aqueous sodium hydroxide or ammonia is added. Precipitates formed are identified. It also lists several anions (carbonate, chloride, iodide, nitrate, sulfate) and their confirmatory chemical tests involving reactions like the formation of gases, precipitates with silver or barium salts, or color changes. The document serves as a reference for students to identify common metal cations and inorganic anions through characteristic chemical reactions.
Interfering radicals in qualitative analysis



Interfering radicals in qualitative analysisrajeeshRajeeshpraj Certain anions like oxalate, tartrate, fluoride, borate, phosphate, and chromate can interfere with the qualitative analysis of cations if not removed. They are eliminated through processes like dry ignition (oxalate), treatment with hydrochloric acid (fluoride, borate), precipitation with zirconyl nitrate (phosphate), and evaporation with hydrochloric acid (chromate). Arsenate is first reduced to arsenite using ammonium iodide before both are eliminated by precipitation with hydrogen sulfide. The order of elimination is oxalate, tartrate, fluoride, borate, phosphate, and arsenate/arsenite to ensure accurate analysis of metal cations.
Reactions Of Metals And Metal Compounds



Reactions Of Metals And Metal Compoundsamr hassaan The document discusses chemical reactions involving metals and their compounds. It explains that metals react with acids to produce salts and hydrogen gas. It also discusses how metals react with oxygen and carbon dioxide to form metal oxides and metal carbonates, which can then further react with acids. The document provides examples of word equations for common metal-acid reactions and identifies the products and reactants in various chemical changes involving metals and their compounds.
Chemistry formula list 2 (Examville.com)



Chemistry formula list 2 (Examville.com)JSlinkyNY 1. The document provides information on the rates of chemical reactions including definitions and methods for calculating rates from graphs.
2. It also summarizes several common chemical reactions like precipitation, combustion, substitution, addition, oxidation and reduction reactions.
3. Details are given for important industrial processes like the Haber, Contact, and Ostwald processes for producing ammonia, sulfuric acid, and nitric acid respectively.
Chapter 8: Reactions in Aqueous Solution



Chapter 8: Reactions in Aqueous SolutionMelissa McDonald This chapter discusses different types of chemical reactions in aqueous solutions. It introduces driving forces that cause reactions, such as formation of a solid, water, or gas. It explains how to predict products using solubility rules and oxidation-reduction reactions when metals react with nonmetals. Reactions are classified into double displacement, acid-base, single replacement, combustion, synthesis, or decomposition reactions based on their driving forces.
OXYGEN



OXYGENUlfa Dewanti The document discusses various oxygen and sulfur compounds. It describes the characteristics of common oxygen groups like oxygen, ozone, hydrogen peroxide and their bonding structures. It also discusses the properties of sulfur and sulfur compounds such as hydrogen sulfide, sulfides, sulfur oxides, sulfuric acid, sulfur salts and sulfur halides. The document provides information on the tendencies and bonding preferences in these oxygen and sulfur compounds.
Naming Compounds



Naming Compoundslallen This document provides an overview of basic chemistry concepts related to naming and writing formulas for ionic compounds. It discusses:
1) How to name ionic compounds by writing the cation first followed by the anion with "-ide" ending, except for polyatomic ions.
2) How to write formulas for ionic compounds by ensuring charges are balanced between cations and anions using subscripts.
3) Special rules for writing formulas involving polyatomic ions and d-block metal cations, which can have multiple oxidation states requiring specifying the oxidation number.
4) How to calculate formula masses of compounds by multiplying the number of atoms of each element by the atomic mass and summing the products.
Single Replacement Reactions



Single Replacement Reactionsduncanpatti Single replacement reactions occur when a metal replaces another metal in a solution. The metal activity series determines which metal is more reactive and able to displace another. Metals higher on the activity series are stronger and can replace metals lower on the list. A metal single replacement reaction involves a metal (A) displacing another metal (B) from a compound (BC) to form a new compound (AC) and leave the displaced metal (B) on its own. Halogens also undergo single replacement reactions where a halogen replaces another halogen in a compound.
Displacement reaction



Displacement reactionsuryacad 1) Displacement reactions can be classified as metal-metal displacement reactions or metal-nonmetal displacement reactions.
2) In metal-metal displacement reactions, more reactive metals displace less reactive metals according to the reactivity series. For example, copper displaces iron when an iron bar is placed in copper sulfate solution.
3) In metal-nonmetal displacement reactions, metals can displace hydrogen from water, with more reactive metals like sodium displacing hydrogen even at room temperature, while less reactive metals like iron only displacing hydrogen when heated.
More Related Content
What's hot (20)
The Periodic Table



The Periodic TableArrehome 1) The document discusses the periodic table and properties of Group 1 elements (alkali metals).
2) Group 1 elements have one electron in their outer shell and form ions with a +1 charge. Their compounds include oxides, chlorides, sulfates and carbonates.
3) Experiments show the reactivity of Group 1 metals increases down the group with francium being the most reactive and reacting explosively with water. The reactions with water produce alkali metal hydroxides and hydrogen gas.
Revision on acid base and salt = with answers



Revision on acid base and salt = with answersMRSMPC This document provides information about chemistry revision on acids, bases and salts. It discusses soluble and insoluble salts such as chlorides, sulphates and nitrates. It also describes methods for preparing soluble and insoluble salts, including the titration and solid acid methods. The document further discusses the preparation of copper(II) sulphate through the reaction of copper(II) oxide with sulphuric acid, and provides chemical tests to identify the copper and sulphate ions.
Solubility



SolubilityLumen Learning Introduces the concepts involved in predicting whether substances are soluble or insoluble. Precipitation (exchange) reactions are also discussed, along with ionic equations and net ionic equations. General Chemistry
Chapter 8 : SALTS



Chapter 8 : SALTSAndromendas Rizal This document discusses the preparation and classification of salts. Salts are formed through the replacement of hydrogen ions in acids by metal ions or ammonium ions. There are two main methods for preparing salts - neutralization and precipitation. Neutralization involves reacting an acid with a metal, alkali, oxide or carbonate to form a soluble salt. Precipitation involves mixing two aqueous solutions of soluble salts to form an insoluble salt precipitate. The document provides examples of preparing various salts such as potassium chloride and lead chloride. It also discusses classifying salts as soluble or insoluble and purifying soluble salts through recrystallization.
Chapter 8 salt part 1



Chapter 8 salt part 1Syaurah Ashikin The document discusses learning outcomes about salts. It defines a salt as a compound formed when a hydrogen ion from an acid is replaced by a metal ion or ammonium ion. Examples of commonly used salts include NaCl, MSG, and CaCO3. Salts can be soluble or insoluble depending on their cation and anion. The document also describes different methods for preparing soluble and insoluble salts, such as titration, evaporation/heating, cooling/crystallization, filtration, and drying.
Qualitative analysis 1



Qualitative analysis 1Mark Selby The basic chemistry you need to understand in order to start your practical work on Qualitative Analysis.
Chemistry prediction of products and workshop homework



Chemistry prediction of products and workshop homeworkWilliard Joshua Jose This document provides an overview of different types of chemical reactions including:
1) Combination/synthesis reactions where elements or compounds combine to form new substances
2) Decomposition reactions where a single compound breaks down into simpler substances
3) Combustion reactions where a substance reacts with oxygen to form oxides
4) Displacement reactions where an element replaces another in a compound
5) Double displacement reactions where ions are swapped between reactants to form new compounds
It then provides a series of practice problems for students to write balanced chemical equations for different laboratory scenarios involving these reaction types.
Chapter 8 Salt part 6



Chapter 8 Salt part 6Syaurah Ashikin The document describes the process and tests used in qualitative analysis to identify salts based on their physical properties, reaction to heat, and tests to detect specific cations and anions. It provides details on observing the color and solubility of salts, conducting gas tests, and using confirmatory tests to identify ions like Fe2+, Fe3+, Pb2+, and NH4+. The qualitative analysis plan involves examining the salt's physical properties, heating it, testing for cations and anions, and then confirming the identities of ions present.
SPM Form 4 Chapter 8 Salt



SPM Form 4 Chapter 8 Saltyuenkei This document summarizes the solubility of different types of salts in water. It states that hydroxides are generally insoluble except for potassium and sodium hydroxide. Oxides are also largely insoluble except for potassium and sodium oxide. Carbonates are more soluble, with sodium, potassium, and ammonium carbonates all soluble. Sulphates and chlorides are also largely soluble, except for a few exceptions like barium and lead salts. Nitrates and salts of sodium and potassium are all soluble in water. It also provides tests to identify different cations and anions in salts.
3. qualitative analysis



3. qualitative analysisPluviose Qualitative analysis is used to identify the cations and anions present in an unknown chemical substance. Cations such as sodium, calcium, and ammonium can be identified using sodium hydroxide and ammonia solutions. Anions like chloride, nitrate, and sulfate can be identified through chemical tests involving silver nitrate, sodium hydroxide with aluminum foil, and barium chloride solutions respectively. These tests produce characteristic precipitates or gas emissions to reveal the ions present. Dilute nitric acid is first added to remove any interfering carbonate ions.
Preciptation reactions 1



Preciptation reactions 1felisapinilla This document describes an experiment on precipitation reactions involving double displacement reactions. A variety of cation and anion solutions were mixed, including AgNO3, Pb(NO3)2, CaCl2, KI, NaCl, NaOH, FeCl3, KNO3, Na2CO3, and CuSO4. Precipitates formed when AgNO3 was mixed with KI (silver iodide), NaCl (silver chloride), CuSO4 (silver sulfate), Na2CO3 (silver carbonate), and FeCl3 (silver chloride). Precipitates also formed when Pb(NO3)2 was mixed with KI (lead iodide), NaCl (lead chloride), Na2
Transition Metals and Complexes, Vanadium and Chromium metal



Transition Metals and Complexes, Vanadium and Chromium metalSidra Javed Shapes and Color of Coordinations Compounds depends upon the Ligands and d-d transition of electrons in the central elements
Redoxhints



RedoxhintsTimothy Welsh This document summarizes important oxidizers and reducers formed in redox reactions under different conditions. It lists common oxidizing agents like MnO4-, Cr2O7-2, and HNO3 that form reduced products like Mn(II), Cr(III), and NO in acid solutions. It also lists common reducers like halide ions, metals, and sulfite ions that form oxidized products like halogens, metal ions, and SO4-2. The document concludes that redox reactions involve electron transfer between oxidizing and reducing agents, and that acidic or basic conditions often indicate a redox reaction will occur.
Test for cat ions an ions



Test for cat ions an ionsShazeel Dhiffushi This document provides information on common cation and anion tests in chemistry. It lists several cations (aluminum, ammonium, calcium, copper, iron, zinc, lead) and their reactions when aqueous sodium hydroxide or ammonia is added. Precipitates formed are identified. It also lists several anions (carbonate, chloride, iodide, nitrate, sulfate) and their confirmatory chemical tests involving reactions like the formation of gases, precipitates with silver or barium salts, or color changes. The document serves as a reference for students to identify common metal cations and inorganic anions through characteristic chemical reactions.
Interfering radicals in qualitative analysis



Interfering radicals in qualitative analysisrajeeshRajeeshpraj Certain anions like oxalate, tartrate, fluoride, borate, phosphate, and chromate can interfere with the qualitative analysis of cations if not removed. They are eliminated through processes like dry ignition (oxalate), treatment with hydrochloric acid (fluoride, borate), precipitation with zirconyl nitrate (phosphate), and evaporation with hydrochloric acid (chromate). Arsenate is first reduced to arsenite using ammonium iodide before both are eliminated by precipitation with hydrogen sulfide. The order of elimination is oxalate, tartrate, fluoride, borate, phosphate, and arsenate/arsenite to ensure accurate analysis of metal cations.
Reactions Of Metals And Metal Compounds



Reactions Of Metals And Metal Compoundsamr hassaan The document discusses chemical reactions involving metals and their compounds. It explains that metals react with acids to produce salts and hydrogen gas. It also discusses how metals react with oxygen and carbon dioxide to form metal oxides and metal carbonates, which can then further react with acids. The document provides examples of word equations for common metal-acid reactions and identifies the products and reactants in various chemical changes involving metals and their compounds.
Chemistry formula list 2 (Examville.com)



Chemistry formula list 2 (Examville.com)JSlinkyNY 1. The document provides information on the rates of chemical reactions including definitions and methods for calculating rates from graphs.
2. It also summarizes several common chemical reactions like precipitation, combustion, substitution, addition, oxidation and reduction reactions.
3. Details are given for important industrial processes like the Haber, Contact, and Ostwald processes for producing ammonia, sulfuric acid, and nitric acid respectively.
Chapter 8: Reactions in Aqueous Solution



Chapter 8: Reactions in Aqueous SolutionMelissa McDonald This chapter discusses different types of chemical reactions in aqueous solutions. It introduces driving forces that cause reactions, such as formation of a solid, water, or gas. It explains how to predict products using solubility rules and oxidation-reduction reactions when metals react with nonmetals. Reactions are classified into double displacement, acid-base, single replacement, combustion, synthesis, or decomposition reactions based on their driving forces.
OXYGEN



OXYGENUlfa Dewanti The document discusses various oxygen and sulfur compounds. It describes the characteristics of common oxygen groups like oxygen, ozone, hydrogen peroxide and their bonding structures. It also discusses the properties of sulfur and sulfur compounds such as hydrogen sulfide, sulfides, sulfur oxides, sulfuric acid, sulfur salts and sulfur halides. The document provides information on the tendencies and bonding preferences in these oxygen and sulfur compounds.
Naming Compounds



Naming Compoundslallen This document provides an overview of basic chemistry concepts related to naming and writing formulas for ionic compounds. It discusses:
1) How to name ionic compounds by writing the cation first followed by the anion with "-ide" ending, except for polyatomic ions.
2) How to write formulas for ionic compounds by ensuring charges are balanced between cations and anions using subscripts.
3) Special rules for writing formulas involving polyatomic ions and d-block metal cations, which can have multiple oxidation states requiring specifying the oxidation number.
4) How to calculate formula masses of compounds by multiplying the number of atoms of each element by the atomic mass and summing the products.
Viewers also liked (20)
Single Replacement Reactions



Single Replacement Reactionsduncanpatti Single replacement reactions occur when a metal replaces another metal in a solution. The metal activity series determines which metal is more reactive and able to displace another. Metals higher on the activity series are stronger and can replace metals lower on the list. A metal single replacement reaction involves a metal (A) displacing another metal (B) from a compound (BC) to form a new compound (AC) and leave the displaced metal (B) on its own. Halogens also undergo single replacement reactions where a halogen replaces another halogen in a compound.
Displacement reaction



Displacement reactionsuryacad 1) Displacement reactions can be classified as metal-metal displacement reactions or metal-nonmetal displacement reactions.
2) In metal-metal displacement reactions, more reactive metals displace less reactive metals according to the reactivity series. For example, copper displaces iron when an iron bar is placed in copper sulfate solution.
3) In metal-nonmetal displacement reactions, metals can displace hydrogen from water, with more reactive metals like sodium displacing hydrogen even at room temperature, while less reactive metals like iron only displacing hydrogen when heated.
Types Of Chemical Reactions



Types Of Chemical ReactionsBen Wildeboer This document discusses a chemistry experiment involving aluminum and copper (II) chloride. The experiment combines aluminum and copper (II) chloride to observe the chemical reaction. The document is titled "Aluminum & Copper II Chloride" and was created by Mr. W.
Different types of chemical reactions(ppt)



Different types of chemical reactions(ppt)utkarshs92 Utkarsh Singh presented on the different types of chemical reactions. There are several types including combination reactions, decomposition reactions, displacement reactions, and double displacement reactions. Combination reactions involve elements or compounds combining to form a new substance. Decomposition reactions involve breaking a substance down into simpler substances. Displacement reactions involve one element replacing another in a compound. Double displacement reactions involve ion exchange between two ionic compounds. Oxidation-reduction reactions involve the transfer of electrons between reactants. Exothermic reactions release heat while endothermic reactions absorb heat from their surroundings.
Chemical equations and reactions



Chemical equations and reactionsTARUN DADWAL This document discusses chemical reactions and equations. It defines chemical reactions as chemical changes where reactants are converted to products. There are five signs that a chemical reaction has occurred: a change in color, evolution of a gas, change in temperature, change in state, or formation of a precipitate. Word equations show the reactants and products using chemical names, while chemical equations use chemical formulas and are balanced according to the law of conservation of mass. The document describes several types of chemical reactions including combination, decomposition, displacement and redox reactions.
Chemical reactions



Chemical reactionsSimran Khirbat Chemical reactions involve chemical changes and the formation of new products. There are four main signs that a chemical reaction has occurred: a change in color, evolution of a gas, change in temperature, or change in state. There are five main types of chemical reactions: combination, decomposition, single displacement, double displacement, and redox. Combination reactions involve reactants combining to form products. Decomposition reactions involve a single reactant breaking down into simpler products. Displacement reactions involve a more reactive element displacing a less reactive one. Redox reactions involve the gain or loss of oxygen.
Balancing and reaction types



Balancing and reaction typesInga Teper Chemical reactions involve reactants transforming into products. There are several types of chemical reactions that can be identified by their reactants and products. These include synthesis reactions where two reactants combine to form one product, decomposition reactions where one reactant breaks down into multiple products, and displacement reactions where one element replaces another in a compound. Balancing chemical equations ensures the same number and type of atoms are on both sides of the reaction.
Acid base reactions



Acid base reactionsknmckee This document defines acids and bases, describing their properties and common uses. Acids have a pH below 7 and taste sour, while bases have a pH above 7 and feel soapy. The document explains that acids and bases react through neutralization, forming less acidic or basic salt mixtures. Common acids include acetic, citric, and sulfuric acids, which are found in vinegar, fruits, and industrial chemicals, while bases are found in soaps, cleaners, and the human body.
Types of Chemical Reaction - Classroom Discussion



Types of Chemical Reaction - Classroom DiscussionJaycris Agnes This document contains notes from a chemistry laboratory class. It includes the chemical formulas for various compounds tested in a quiz, as well as sections on different types of chemical reactions like combination, decomposition, single displacement and double displacement reactions. Examples are provided for each type of reaction. Safety precautions for the laboratory are emphasized.
Diffusion lesson



Diffusion lessonandrewpickett This document discusses diffusion in particles including solids, liquids, and gases. It provides examples of gas and liquid diffusion, describes the processes of diffusion, and explains how diffusion differs between gases and liquids. Gas particles move freely and quickly without bonds, allowing gas diffusion to occur rapidly, while liquid particles still move freely but more slowly with bonds between them, resulting in slower liquid diffusion.
Resistivity and resistance.ppt



Resistivity and resistance.pptmrmeredith Resistivity and resistance are affected by several factors. The resistivity of a material determines how well it conducts electricity. Resistance increases as resistivity, length, or decreases in area increase. Factors like temperature, material properties, and geometry impact resistance and resistivity. Thermistors have resistance that changes with temperature, allowing them to act as temperature sensors when calibrated in a circuit. Superconductors have zero resistance below a critical temperature threshold, enabling applications like power lines and electromagnets.
Chemical reactions



Chemical reactionskundana This document discusses different types of chemical reactions. It describes combination reactions where two reactants form one product, decomposition reactions where a single compound breaks down into simpler substances through thermal, electrolytic, or photochemical means, and displacement and double displacement reactions. Key examples are provided for combination reactions like zinc and iodine forming zinc iodide, and decomposition reactions like silver chloride breaking down into silver and chlorine gas when exposed to sunlight.
Types of Reactions: Decomposition and Single Replacement



Types of Reactions: Decomposition and Single Replacementalchemist The document describes the five main types of chemical equations:
1) Synthesis reactions - Combining two or more substances to form one product
2) Decomposition reactions - One substance breaking down into two or more products
3) Single replacement reactions - An element replaces another in a compound
4) Double replacement reactions - Ionic compounds exchange ions to form new compounds
5) Combustion reactions - A substance reacts with oxygen to form oxides
Examples of each type of reaction are provided, along with guidance on writing and balancing chemical equations.
Bernoulli’s principle



Bernoulli’s principleSheeRa Aya Daniel Bernoulli discovered Bernoulli's principle in the 1700s through experiments observing water flow. Bernoulli's principle states that as the speed of a fluid increases, the pressure decreases. It explains that the pressure is lowest where the flow speed is highest. Some applications of this principle are how aircraft wings generate lift through differences in air pressure above and below the wing, and how ventilation works through higher pressure pushing lower pressure areas.
Electricity: Resistance



Electricity: ResistancePamantasan ng Lungsod ng Maynila The document discusses electricity and resistance. It defines resistance as the ratio of voltage to current and explains that resistance depends on a material's resistivity, length, and cross-sectional area. It introduces Georg Ohm and Ohm's Law, which states that voltage and current are directly proportional. Resistors are circuit elements that produce voltage proportional to current according to Ohm's Law. Conductors contain movable electric charges like electrons that allow current to flow. Insulators contain fewer movable charges and resist current. The document contrasts alternating and direct current, noting that DC involves a constant unidirectional flow of charge.
Chemical Reactions



Chemical ReactionsOhMiss A chemical reaction involves the transformation of reactants into different products through rearrangement of atoms. Chemical reactions conserve mass as atoms are not destroyed or created, but instead are reorganized into new substances. Balancing chemical equations ensures the same number and type of atoms are on both sides of the reaction.
Acid rain greenhouse effect



Acid rain greenhouse effectAshrith N Shetty Acid rain is caused by sulfur dioxide and nitrogen oxides reacting with water and oxygen in the atmosphere to form acids. The main sources of these pollutants are burning fossil fuels like coal. Acid rain damages plants, soils, aquatic animals and buildings. To prevent acid rain and the greenhouse effect, we must reduce coal use and emissions of carbon dioxide and methane by switching to renewable energy and improving energy efficiency.
identification of cations and anions present in toothpaste



identification of cations and anions present in toothpasteM Sai Sankharan The document describes an experiment to identify the cations and anions present in toothpaste. Key findings include:
- Tests were conducted on samples of toothpaste using chemical reagents. Precipitates formed indicating the presence of calcium, magnesium, phosphate, carbonate, and iodide ions.
- Additional components found in toothpaste include abrasives like silica, fluoride compounds, and surfactants. Other common ingredients are antibacterial agents, flavorings, and remineralizers.
- Toothpaste aims to promote oral hygiene through abrasion of plaque, delivery of fluoride, and foaming which helps distribute the paste. While not intended for swallowing, small amounts accidentally ingested are generally
Electrolysis revision



Electrolysis revisionsue forshaw This document provides information about electrolysis. It discusses:
1. How electrolysis can be used to split up ionic compounds by melting them or dissolving them in water. Positive ions move to the negative cathode and negative ions move to the positive anode.
2. The rules for what products are formed at the electrodes during electrolysis of molten ionic compounds and ionic solutions. Hydrogen, metals, halogens, oxygen may be produced depending on the ions present.
3. Industrial applications of electrolysis including producing chlorine and sodium hydroxide from salt water, and purifying copper.
Diffusion of gases



Diffusion of gasesShubhanshu Jain Diffusion is the movement and mixing of gas particles. It occurs more quickly for gases than liquids and at higher temperatures. In one experiment, ammonia and hydrochloric acid gases diffuse towards each other in a sealed tube and react to form ammonium chloride particles. Ammonia particles diffuse faster because they have a lower molar mass than hydrochloric acid particles. Graham's law states that the rate of effusion, or movement through a small hole, is inversely proportional to the square root of a gas's molar mass.
Similar to Lab 4 (20)
Types of Reactions PPT.pdf_١١٢٢٠٠٠٤.pptx



Types of Reactions PPT.pdf_١١٢٢٠٠٠٤.pptxsalehalgabri02 There are five main types of chemical reactions: synthesis, decomposition, single replacement, double replacement, and combustion. A chemical reaction occurs when atoms bond together to form new compounds or when compounds separate to form other compounds. Chemical reactions can be identified by certain characteristics like gas produced, light produced, temperature change, color change, or precipitate formed.
Chapter 4 Lecture- Solution Stoich



Chapter 4 Lecture- Solution StoichMary Beth Smith This document provides information on various chemistry concepts related to solutions and reactions in aqueous solutions. It defines key terms like electrolytes, nonelectrolytes, dissociation, and precipitation reactions. It also discusses acid-base reactions and neutralization reactions. Oxidation-reduction reactions and displacement reactions are introduced. Molarity is defined as a way to quantify concentration in solutions.
Chpt 9 part ii - types of reactions 031604



Chpt 9 part ii - types of reactions 031604phspsquires This document provides an overview of common types of chemical reactions including synthesis, decomposition, single replacement, double replacement, acid-base neutralization, and combustion reactions. Examples of each type of reaction are given along with general formulas. Key concepts covered include ions, oxidation-reduction reactions, and predicting products of different reaction types.
Ch4 Reactions in Aqueous Solution (updated)



Ch4 Reactions in Aqueous Solution (updated)Sa'ib J. Khouri A document discusses various topics relating to chemistry solutions including:
1) The definition of solutions, solvents, and solutes. A solution is a homogeneous mixture of substances where the solute is the smaller component dissolved in the solvent.
2) Properties of aqueous solutions including that electrolytes can conduct electricity while nonelectrolytes cannot. Strong electrolytes dissociate completely while weak electrolytes only partially dissociate.
3) Reactions involving solutions such as precipitation reactions, acid-base reactions, and redox reactions. Precipitation occurs when an insoluble solid forms. Acid-base reactions involve acids and bases reacting to form water and a salt. Redox reactions involve the transfer of electrons
Chemical equations best



Chemical equations bestJeff Kalember 1. The document discusses chemical equations, including word equations, balanced equations, and how to interpret and balance chemical equations.
2. It explains the key parts of a chemical equation including reactants, products, phases (solid, liquid, gas), and how to write ionic equations.
3. The document also covers different types of chemical reactions like synthesis, decomposition, single replacement, and double replacement reactions and how to predict products.
Ch4 Reactions in Aqueous Solution



Ch4 Reactions in Aqueous SolutionSa'ib J. Khouri This document discusses various topics related to aqueous solutions and reactions. It begins by defining key terms like solute, solvent, electrolyte and providing examples. It then covers properties of aqueous solutions such as conductivity. Various acid-base reactions and concepts are explained like Brønsted-Lowry acids and bases, neutralization reactions. Oxidation-reduction reactions and oxidation numbers are also discussed. Finally, the document covers concentration of solutions and calculations involving molarity, dilution and preparation of solutions.
introduction to puckers.pptx



introduction to puckers.pptxitzkuu01 1) The document discusses various types of evidence that indicate a chemical reaction has occurred, including temperature change, gas formation, color change, and precipitate formation.
2) It describes word equations, skeleton equations, and how to translate between the two. Balancing equations requires using coefficients to satisfy the law of conservation of mass.
3) Key characteristics of the main types of chemical reactions - synthesis, decomposition, single replacement, double replacement, and combustion - are outlined.
Introduction_to_Chemical_Reactions_2011-2012.ppt



Introduction_to_Chemical_Reactions_2011-2012.pptVicky570089 1) A chemical reaction is a process where one or more substances are destroyed and one or more new substances are created.
2) There are five main types of chemical reactions: single replacement, double replacement, synthesis, decomposition, and combustion.
3) Evidence for a chemical reaction includes evolution of light or heat, temperature changes, formation of gases or precipitates, and color changes.
Introduction_to_Chemical_Reactions_2011-2012.ppt



Introduction_to_Chemical_Reactions_2011-2012.pptMuneeba Khalid The document provides an introduction to chemical reactions, including definitions, parts of reactions, types of reactions, and evidence of reactions. It explains that a chemical reaction is a process where reactants are destroyed and products are formed, with the following key points:
- Reactants undergo chemical change to form products, as indicated by the yield arrow (→).
- Chemical equations must obey the law of conservation of mass, with the same number and type of atoms on both sides of the reaction.
- The five main types of chemical reactions are synthesis, decomposition, single replacement, double replacement, and combustion.
- Several tests can indicate if a chemical reaction has occurred, such as gas evolution, temperature change, color change,
Chemical_Reactions.ppt



Chemical_Reactions.pptJerwinNicolastico 1) A chemical reaction is a process where one or more substances are destroyed and one or more new substances are created.
2) There are five main types of chemical reactions: single replacement, double replacement, synthesis, decomposition, and combustion.
3) Evidence for a chemical reaction includes evolution of light or heat, temperature changes, formation of gases or precipitates, and color changes.
Introduction to Chemical Reaction and types



Introduction to Chemical Reaction and typestriptiaggarwal29 1) A chemical reaction is a process where one or more substances are destroyed and one or more new substances are created.
2) There are five main types of chemical reactions: single replacement, double replacement, synthesis, decomposition, and combustion.
3) Evidence for a chemical reaction includes evolution of light or heat, temperature changes, formation of gases or precipitates, and color changes.
AP Chemistry Chapter 4 Outline



AP Chemistry Chapter 4 OutlineJane Hamze This document summarizes key concepts in solution chemistry and stoichiometry, including:
1) Solutions, electrolytes, dissociation, and precipitation reactions are discussed. Strong and weak electrolytes are defined.
2) Acid-base reactions such as neutralization and gas-forming reactions are covered. Oxidation-reduction reactions and oxidation numbers are also introduced.
3) Concepts like molarity, dilution, and titration are explained as methods to quantify concentrations in solutions and chemical reactions.
Ch04 outline



Ch04 outlineAP_Chem This document summarizes key concepts in solution chemistry and stoichiometry, including:
1) Solutions, electrolytes, dissociation, and precipitation reactions are discussed. Strong and weak electrolytes are defined.
2) Acid-base reactions such as neutralization and gas-forming reactions are covered. Oxidation-reduction reactions and displacement reactions are also summarized.
3) Concepts including molarity, dilution, and titration reactions are introduced for quantitative chemical calculations.
An Introduction to Chemical Reactions.ppt



An Introduction to Chemical Reactions.pptkirstenfajardo18 It is a chemical change in which one or more substances are destroyed and one or more new substances are created.
Evidence for Chemical Reaction.
Translating Word Equations to Skeleton Equations.
Chem65chapter7.ppt



Chem65chapter7.pptAliceCRivera This document provides an outline and overview of key concepts in chemical reactions from a chemistry textbook. It discusses topics like chemical equations, balancing equations, types of chemical reactions including synthesis, decomposition, and displacement reactions. It also covers activity series, aqueous reactions involving precipitation and neutralization, and gas forming reactions. Examples are provided to illustrate concepts like writing and balancing equations.
Science 10th Class 



Science 10th Class Rahul Thakur This document provides information on stoichiometry, which involves using mole ratios from balanced chemical equations to calculate mass relationships between substances in a chemical reaction. It outlines the steps to solve stoichiometry problems, which include writing a balanced equation, identifying known and unknown quantities, setting up mole ratio conversion factors between moles of reactants and products, and checking the answer. Key concepts discussed include the mole ratio from coefficients in a balanced equation, molar mass to convert between moles and grams, and the molar volume used to calculate liters of gas at standard temperature and pressure.
Chemical Reactions



Chemical ReactionsCurrituck County High School 1. The document discusses chemical reactions, including what makes something a chemical reaction, different ways to describe reactions using word equations, chemical equations, and balanced equations.
2. It describes the main types of chemical reactions - synthesis, decomposition, single replacement, double replacement, and combustion reactions - and provides examples of each.
3. Guidelines are given for writing balanced chemical equations, including identifying the reaction type and using coefficients to balance the mass on each side.
Chemistry - Chp 11 - Chemical Reactions - PowerPoint



Chemistry - Chp 11 - Chemical Reactions - PowerPointMr. Walajtys This document summarizes key points from a chemistry textbook chapter on chemical reactions. It describes the five major types of chemical reactions: combination, decomposition, single replacement, double replacement, and combustion. It explains how to identify the type of reaction based on the reactants and how to write and balance chemical equations. It also discusses precipitation reactions in double replacement reactions in aqueous solution and the activity series for predicting single replacement reactions.
More from dluetgens (20)
Stoich w s-sr_and_dr with key



Stoich w s-sr_and_dr with keydluetgens This document contains 4 stoichiometry problems involving single and double replacement reactions. For each problem, students are asked to write balanced, total ionic, and net ionic equations and use them to calculate the amount of product formed given the amount of a reactant. The problems include reactions between iron and silver nitrate, tin(II) chloride and sodium sulfide, magnesium and zinc phosphate, and aluminum nitrate and sodium hydroxide.
Lab #5 lab sample



Lab #5 lab sampledluetgens The document describes a lab experiment comparing the reactions of different metals with hydrochloric acid. Students observed the reactions of zinc, magnesium, copper, and aluminum with HCl of varying concentrations. Zinc and magnesium reacted vigorously while copper did not react at all. The purpose was to model oxidation-reduction reactions and construct equations to summarize the single replacement reactions. Students analyzed the reactions in terms of atoms becoming ions, oxidation states changing, and the energy changes involved. Reactions that produced heat were determined to be exothermic.
Short version glycolysis



Short version glycolysisdluetgens Glycolysis is a 10 step process that converts glucose into pyruvate, producing a net yield of 2 ATP per glucose molecule. Key steps include:
1) Phosphorylation of glucose to glucose-6-phosphate via hexokinase.
2) Isomerization of glucose-6-phosphate to fructose-6-phosphate via phosphoglucose isomerase.
3) Phosphorylation of fructose-6-phosphate to fructose-1,6-bisphosphate via phosphofructokinase.
4) Cleavage of fructose-1,6-bisphosphate into two 3-carbon molecules, one of which continues through glycol
Part one ycta pac 2010



Part one ycta pac 2010dluetgens The YCTA PAC engaged in several political activities prior to 2009 including phone campaigns urging legislators to minimize education cuts. In 2010, the YCTA recruited and interviewed school board candidates, ultimately endorsing four candidates believed to balance the board. Site representatives distributed information about endorsed candidates to members despite district restrictions on communications. The YCTA and CTA visited school sites to meet with members but the district initially resisted, requiring time to approve visits.
Part two ycta pac 2010



Part two ycta pac 2010dluetgens The document summarizes events from November 2010 related to a local teachers association's (YCTA) election efforts and results. It discusses:
1) The YCTA holding an open house event for candidates and the community.
2) Promotional efforts for a rally held the day before the election to give it one last effort.
3) Displays of mobile banners and balloons at schools on election day to get out the vote.
4) Announcing the results of the school board election and expressing happiness that dedicated candidates were elected.
Protein synthesis



Protein synthesisdluetgens 1. DNA contains the genetic code for proteins in the form of sequences of nucleotides. RNA carries copies of the DNA code to direct protein synthesis.
2. During transcription, RNA polymerase uses one DNA strand as a template to assemble an mRNA copy of the genetic code.
3. During translation, mRNA binds to ribosomes where tRNA molecules match codons on mRNA to the anticodons carrying amino acids, assembling proteins based on the code.
Vsepr



Vseprdluetgens The document discusses the VSEPR (Valence Shell Electron Pair Repulsion) model for understanding molecular geometry. It explains that chemical bonds form when collections of atoms are more stable with lower energy than separate atoms. The VSEPR model uses electron pairs around central atoms to predict molecular shapes and bond angles based on minimizing electron pair repulsions. Multiple examples are provided to illustrate VSEPR principles for molecules with different numbers of electron pairs.
VSEPR



VSEPRdluetgens The document discusses the VSEPR (Valence Shell Electron Pair Repulsion) model for understanding molecular geometry. It explains that chemical bonds form when collections of atoms are more stable and lower in energy than separate atoms. The VSEPR model uses electron pairs around central atoms to predict molecular shapes and bond angles based on minimizing electron pair repulsions. Hybrid orbital theory is also introduced to explain how atomic orbitals combine to form hybrid orbitals that are better suited for bonding arrangements.
Lab 8 atomic structure



Lab 8 atomic structuredluetgens The document provides a history of the development of atomic structure models from ancient Greek philosophers' ideas of indivisible atoms to the modern quantum mechanical model. It describes key experiments and findings such as Thomson's discovery of electrons, Rutherford's gold foil experiment, and Bohr's model of electron orbits that led to modern atomic theory. The emission spectra of elements provided evidence that electrons exist in specific energy levels and orbitals within atoms.
Lab 9 atomic structure



Lab 9 atomic structuredluetgens The document provides a history of the development of atomic structure models from ancient Greek philosophers' ideas of indivisible atoms to the modern quantum mechanical model. It describes key experiments and findings such as Thomson's discovery of electrons, Rutherford's gold foil experiment, and Bohr's model of electron orbits that led to modern atomic theory. The emission spectra of elements provided evidence that electrons exist in specific energy levels and orbitals within atoms.
Glycolysis And Fermentation



Glycolysis And Fermentationdluetgens Cellular respiration involves three main stages: glycolysis, the Krebs cycle, and the electron transport chain. [1] Glycolysis takes place in the cytosol and involves the breakdown of glucose into pyruvate, producing a small amount of ATP. [2] Pyruvate then enters the mitochondrion, where it is further oxidized in the Krebs cycle. [3] Electrons are transferred in the electron transport chain to produce most of the cell's ATP through oxidative phosphorylation.
Carbohydrate Metabolism



Carbohydrate Metabolismdluetgens The document summarizes key concepts related to cellular respiration. It discusses the four main stages: glycolysis, formation of acetyl-CoA, the citric acid cycle, and the electron transport chain. Glycolysis breaks down glucose into pyruvate, generating a small amount of ATP. In later stages, pyruvate fully oxidizes, producing much more ATP through oxidative phosphorylation as electrons are passed through the electron transport chain. The fate of pyruvate depends on whether oxygen is present, determining whether aerobic or anaerobic respiration occurs.
Earthquakes



Earthquakesdluetgens Earthquakes occur along plate boundaries due to the buildup and sudden release of energy from shifting tectonic plates. When plates lock, potential energy builds until released as seismic waves that propagate outward from the earthquake focus. Most earthquakes occur along oceanic and continental plate edges or along faults like normal, reverse, and transform boundaries. P and S waves are the primary seismic waves, with P waves traveling faster and S waves causing the shaking felt during quakes. Earthquake magnitude measures the energy released using the Richter scale, while intensity qualitatively describes the shaking effects on a place using the Mercalli scale.
Plate Tectonics



Plate Tectonicsdluetgens The document discusses plate tectonics and the evidence that supports the theory of continental drift and plate tectonics. It describes how early scientists like Wegener proposed that the continents were once joined together in a supercontinent called Pangaea based on matching coastlines and fossil evidence. Later, developments in technology like sonar and sea floor drilling provided further evidence of seafloor spreading at mid-ocean ridges, supporting the modern theory of plate tectonics. The earth's crust is broken into plates that move relative to each other at different boundary types, including divergent boundaries where new crust is created and convergent boundaries where plates collide.
chem Lab 5



chem Lab 5dluetgens Magnesium reacted immediately with hydrochloric acid, bubbling rapidly until fully dissolved. Zinc reacted more slowly with bubbling. Aluminum reacted slowly only with the stronger acid. Copper did not react at all. This is because magnesium, zinc and aluminum are more reactive than hydrogen in the acid, replacing it to form magnesium chloride, hydrogen gas and electrons in a single replacement reaction. Copper is less reactive than hydrogen and did not replace it.
Abc Kinetics



Abc Kineticsdluetgens The document discusses kinetics and reaction rates. It defines kinetics as the study of reaction rates, and explains that thermodynamics determines if a reaction will occur but not how fast. It then covers factors that affect reaction rates like temperature, concentration, and catalysts. The document provides examples of calculating rates from concentration data and determining rate laws from experimental results. It also introduces integrated rate laws and how to determine reaction order from different rate law equations.
Lab 3



Lab 3dluetgens This lab experiment investigated how the concentration and conductivity of a sodium chloride solution changed as sodium chloride was added incrementally. The solution's conductivity increased with each addition as the number of ions increased, until the fifth addition when undissolved salt remained, indicating saturation. The goal was to prepare saturated solutions of various ionic compounds and determine their concentrations using Ksp calculations and measurements.
Ips Lab 2.2.Dl.



Ips Lab 2.2.Dl.dluetgens This document discusses the phases of water and the particle model that explains the behavior of water. It notes that water drops are high and round, and puts forth the claim that this shape can be explained by the attractions between water particles. It provides evidence from observations that water particles must be attracted to each other through experiments showing how water drops behave when brought near each other or pulled with a toothpick. The document concludes that the round shape of water drops demonstrates the attractive forces between water particles hold the drop up against the force of gravity.
Lab 3



Lab 3dluetgens This lab experiment explored how the concentration and conductivity of a sodium chloride solution changed as sodium chloride was added incrementally. The solution's conductivity increased with each addition as the number of ions increased in the fixed volume of water. Eventually, adding more sodium chloride no longer increased the conductivity, indicating the solution had reached saturation. The saturated solution was in dynamic equilibrium, with ions continuously dissolving from and crystallizing onto the solid.
Solids, Liquids And Gases.Dl



Solids, Liquids And Gases.Dldluetgens Solids, liquids, and gases are distinguished by their physical properties. Solids maintain a fixed shape and volume, as seen with frozen fruit that keeps its shape when frozen. Liquids take the shape of their container but maintain a constant volume, like water in a fish bowl. Gases fill their container completely and can be easily compressed into a smaller volume, as with helium in a balloon.
Recently uploaded (20)
THE BIG TEN BIOPHARMACEUTICAL MNCs: GLOBAL CAPABILITY CENTERS IN INDIA



THE BIG TEN BIOPHARMACEUTICAL MNCs: GLOBAL CAPABILITY CENTERS IN INDIASrivaanchi Nathan This business intelligence report, "The Big Ten Biopharmaceutical MNCs: Global Capability Centers in India", provides an in-depth analysis of the operations and contributions of the Global Capability Centers (GCCs) of ten leading biopharmaceutical multinational corporations in India. The report covers AstraZeneca, Bayer, Bristol Myers Squibb, GlaxoSmithKline (GSK), Novartis, Sanofi, Roche, Pfizer, Novo Nordisk, and Eli Lilly. In this report each company's GCC is profiled with details on location, workforce size, investment, and the strategic roles these centers play in global business operations, research and development, and information technology and digital innovation.
Leadership u automatizaciji: RPA priče iz prakse!



Leadership u automatizaciji: RPA priče iz prakse!UiPathCommunity Dobrodošli na "AI Powered Automation Leadership Talks", online događaj koji okuplja senior lidere i menadžere iz različitih industrija kako bi podelili svoja iskustva, izazove i strategije u oblasti RPA (Robotic Process Automation). Ovaj događaj pruža priliku da zavirite u način razmišljanja ljudi koji donose ključne odluke u automatizaciji i liderstvu.
📕 Kroz panel diskusiju sa tri izuzetna stručnjaka, istražićemo:
Kako uspešno započeti i skalirati RPA projekte u organizacijama.
Koji su najveći izazovi u implementaciji RPA-a i kako ih prevazići.
Na koje načine automatizacija menja radne procese i pomaže timovima da ostvare više.
Bez obzira na vaše iskustvo sa UiPath-om ili RPA uopšte, ovaj događaj je osmišljen kako bi bio koristan svima – od menadžera do tehničkih lidera, i svima koji žele da unaprede svoje razumevanje automatizacije.
Pridružite nam se i iskoristite ovu jedinstvenu priliku da naučite od onih koji vode automatizaciju u svojim organizacijama. Pripremite svoja pitanja i inspiraciju za sledeće korake u vašoj RPA strategiji!
Webinar: LF Energy GEISA: Addressing edge interoperability at the meter



Webinar: LF Energy GEISA: Addressing edge interoperability at the meterDanBrown980551 This webinar will introduce the Grid Edge Security and Interoperability Alliance, or GEISA, an effort within LF Energy to address application interoperability at the very edge of the utility network: meters and other distribution automation devices. Over the last decade platform manufacturers have introduced the ability to run applications on electricity meters and other edge devices. Unfortunately, while many of these efforts have been built on Linux, they haven’t been interoperable. APIs and execution environment have varied from one manufacturer to the next making it impossible for utilities to obtain applications that they can run across a fleet of different devices. For utilities that want to minimize their supply chain risk by obtaining equipment from multiple suppliers, they are forced to run and maintain multiple separate management systems. Applications available for one device may need to be ported to run on another, or they may not be available at all.
GEISA addresses this by creating a vendor neutral specification for utility edge computing environments. This webinar will discuss why GEISA is important to utilities, the specific issues GEISA will solve and the new opportunities it creates for utilities, platform vendors, and application vendors.
NSFW AI Chatbot Development Costs: What You Need to Know



NSFW AI Chatbot Development Costs: What You Need to KnowSoulmaite Are you considering building an NSFW AI chatbot ?Understanding the costs involved is crucial before starting your project. This PDF explores the key cost factors, including AI model customization, API integration, content filtering systems, and ongoing maintenance expenses. Learn how different pricing models impact the development budget and discover cost-saving strategies without compromising quality.
Revolutionizing Field Service: How LLMs Are Powering Smarter Knowledge Access...



Revolutionizing Field Service: How LLMs Are Powering Smarter Knowledge Access...Earley Information Science Revolutionizing Field Service with LLM-Powered Knowledge Management
Field service technicians need instant access to accurate repair information, but outdated knowledge systems often create frustrating delays. Large Language Models (LLMs) are changing the game—enhancing knowledge retrieval, streamlining troubleshooting, and reducing technician dependency on senior staff.
In this webinar, Seth Earley and industry experts Sanjay Mehta, and Heather Eisenbraun explore how LLMs and Retrieval-Augmented Generation (RAG) are transforming field service operations. Discover how AI-powered knowledge management is improving efficiency, reducing downtime, and elevating service quality.
LLMs for Instant Knowledge Retrieval – How AI-driven search dramatically cuts troubleshooting time.
Structured Data & AI – Why high-quality, organized knowledge is essential for LLM success.
Real-World Implementation – Lessons from deploying LLM-powered knowledge tools in field service.
Business Impact – How AI reduces service delays, optimizes workflows, and enhances technician productivity.
Empower your field service teams with AI-driven knowledge access. Watch the webinar to see how LLMs are revolutionizing service efficiency.
DealBook of Ukraine: 2025 edition | AVentures Capital



DealBook of Ukraine: 2025 edition | AVentures CapitalYevgen Sysoyev The DealBook is our annual overview of the Ukrainian tech investment industry. This edition comprehensively covers the full year 2024 and the first deals of 2025.
Dev Dives: Unlock the future of automation with UiPath Agent Builder



Dev Dives: Unlock the future of automation with UiPath Agent BuilderUiPathCommunity This webinar will offer you a first look at the powerful capabilities of UiPath Agent Builder, designed to streamline your automation processes and enhance your workflow efficiency.
📕 During the session, you will:
- Discover how to build agents with low-code experience, making it accessible for both developers and business users.
- Learn how to leverage automations and activities as tools within your agents, enabling them to handle complex and dynamic workflows.
- Gain insights into the AI Trust Layer, which provides robust management and monitoring capabilities, ensuring trust and transparency in your automation processes.
- See how agents can be deployed and integrated with your existing UiPath cloud and Studio environments.
👨🏫 Speaker:
Zach Eslami, Sr. Manager, Product Management Director, UiPath
⏩ Register for our upcoming Dev Dives March session:
Unleash the power of macOS Automation with UiPath
👉 AMER: https://bit.ly/Dev_Dives_AMER_March
👉 EMEA & APJ:https://bit.ly/Dev_Dives_EMEA_APJ_March
This session was streamed live on February 27, 2025, 15:00 GMT.
Check out future Dev Dives 2025 sessions at:
🚩 https://bit.ly/Dev_Dives_2025
Not a Kubernetes fan? The state of PaaS in 2025



Not a Kubernetes fan? The state of PaaS in 2025Anthony Dahanne Kubernetes won the containers orchestration war. But has it made deploying your apps easier?
Let's explore some of Kubernetes extensive app developer tooling, but mainly what the PaaS space looks like in 2025; 18 years after Heroku made it popular.
Is Heroku still around? What about Cloud Foundry?
And what are those new comers (fly.io, railway, porter.sh, etc.) worth?
Did the Cloud giants replace them all?
William Maclyn Murphy McRae - A Seasoned Professional Renowned



William Maclyn Murphy McRae - A Seasoned Professional RenownedWilliam Maclyn Murphy McRae William Maclyn Murphy McRae, a logistics expert with 9+ years of experience, is known for optimizing supply chain operations and consistently exceeding industry standards. His strategic approach, combined with hands-on execution, has streamlined distribution processes, reduced lead times, and consistently delivered exceptional results.
SB7 Mobile Ltd: Simplified & Secure Services



SB7 Mobile Ltd: Simplified & Secure ServicesReuben Jasper SB7 Mobile Ltd is enhancing customer experience by improving support accessibility, billing transparency, and security. The company has strengthened payment authorization, simplified unsubscription, and expanded customer service channels to address common concerns.
Caching for Performance Masterclass: Caching at Scale



Caching for Performance Masterclass: Caching at ScaleScyllaDB Weighing caching considerations for use cases with different technical requirements and growth expectations.
- Request coalescing
- Negative sharding
- Rate limiting
- Sharding and scaling
Data-Driven Public Safety: Reliable Data When Every Second Counts



Data-Driven Public Safety: Reliable Data When Every Second CountsSafe Software When every second counts, you need access to data you can trust. In this webinar, we’ll explore how FME empowers public safety services to streamline their operations and safeguard communities. This session will showcase workflow examples that public safety teams leverage every day.
We’ll cover real-world use cases and demo workflows, including:
Automating Police Traffic Stop Compliance: Learn how the City of Fremont meets traffic stop data standards by automating QA/QC processes, generating error reports – saving over 2,800 hours annually on manual tasks.
Anonymizing Crime Data: Discover how cities protect citizen privacy while enabling transparent and trustworthy open data sharing.
Next Gen 9-1-1 Integration: Explore how Santa Clara County supports the transition to digital emergency response systems for faster, more accurate dispatching, including automated schema mapping for address standardization.
Extreme Heat Alerts: See how FME supports disaster risk management by automating the delivery of extreme heat alerts for proactive emergency response.
Our goal is to provide practical workflows and actionable steps you can implement right away. Plus, we’ll provide quick steps to find more information about our public safety subscription for Police, Fire Departments, EMS, HAZMAT teams, and more.
Whether you’re in a call center, on the ground, or managing operations, this webinar is crafted to help you leverage data to make informed, timely decisions that matter most.
Quantum Computing Quick Research Guide by Arthur Morgan



Quantum Computing Quick Research Guide by Arthur MorganArthur Morgan This is a Quick Research Guide (QRG).
QRGs include the following:
- A brief, high-level overview of the QRG topic.
- A milestone timeline for the QRG topic.
- Links to various free online resource materials to provide a deeper dive into the QRG topic.
- Conclusion and a recommendation for at least two books available in the SJPL system on the QRG topic.
QRGs planned for the series:
- Artificial Intelligence QRG
- Quantum Computing QRG
- Big Data Analytics QRG (coming 2025)
- Spacecraft Guidance, Navigation & Control QRG (coming 2026)
- UK Home Computing & The Birth of ARM QRG (coming 2027)
Any questions or comments?
- Please contact Arthur Morgan at art_morgan@att.net.
100% human made.
Benchmark Testing Demystified: Your Roadmap to Peak Performance



Benchmark Testing Demystified: Your Roadmap to Peak PerformanceShubham Joshi Benchmark testing is the cornerstone of understanding your system’s performance, and this guide breaks it down step-by-step. Learn how to design tests that simulate real-world conditions, measure key performance metrics, and interpret results effectively. This comprehensive roadmap covers everything from selecting the right tools to creating repeatable tests that help identify bottlenecks and optimize resource usage. Whether you're dealing with web applications, mobile apps, or enterprise software, this guide offers practical tips and real-life examples to ensure your system runs at peak efficiency.
16 KALALU🕉️🏇🪓🪈🏹👑👑👑👑👑🤴🦁💿✈️🛸🚀🪩🥏🍥 APARAMAHASAHASRA SIMHAMAHANKALKIADIPARASAKTIBH...



16 KALALU🕉️🏇🪓🪈🏹👑👑👑👑👑🤴🦁💿✈️🛸🚀🪩🥏🍥 APARAMAHASAHASRA SIMHAMAHANKALKIADIPARASAKTIBH...IT Industry 🕉️🕺🌎🌌🦸👑👑👑👑👑🤴💝🎉🏆✈️🛸🚀🪩🥏💿🍥🏹🪈🪓🏇 BHAGWAN SRI RAMA SIMHA OMKARAM SRI SRI KING VISNU KALKI SRI KRISNA PARAMATMA SRI SRI KING VISNU SARAT KRISNA PARAMATMA SRI SRI KING ADISIMHA APARAMAHASAHASRA SAHASRASAMASTA SAHASRA SAMASTA APARAMAHASAHASRA BHARGAVA SIMHA TRINETRA APARAMAHASAHASRA SIMHAMAHANKALI ADIPARASAKTI KVADIPARASAKTI ANEKASAHASRA MAHA INFINITY SIMHA SAHASRA AVATARAMULU SRI SRI KING VISNU SUPREME GODS HEADS KING VISNU SUPREME ALFAONKA SAHASRA SAMASTA APARAMAHASAHASRA BHARGAVA SIMHA TRINETRA APARAMAHASAHASRA SIMHAMAHANKALI ADIPARASAKTI KVADIPARASAKTI VAARE SRI SRI KING VISNU KAVERI VEERA BHARAT BHUSHAN MR.KALKIKINGSUPREMEGODSHEADS MEGA KALKI ROBO ALIENS KING SUPREME GODS HEADS KING ALFA SUPREMO 16 KALALU !! 🪓🏇🪈🏹🍥💿🥏🪩🚀🛸✈️🏆🎉💝🤴👑👑👑👑👑🦸🕺🌌🌎🕉️
Caching for Performance Masterclass: Caching Strategies



Caching for Performance Masterclass: Caching StrategiesScyllaDB Exploring the tradeoffs of common caching strategies – and a look at the architectural differences.
- Which strategies exist
- When to apply different strategies
- ScyllaDB cache design
Transcript: AI in publishing: Your questions answered - Tech Forum 2025



Transcript: AI in publishing: Your questions answered - Tech Forum 2025BookNet Canada George Walkley, a publishing veteran and leading authority on AI applications, joins us for a follow-up to his presentation "Applying AI to publishing: A balanced and ethical approach". George gives a brief overview of developments since that presentation and answers attendees' pressing questions about AI’s impact and potential applications in the book industry.
Link to recording and presentation slides: https://bnctechforum.ca/sessions/ai-in-publishing-your-questions-answered/
Presented by BookNet Canada on February 20, 2025 with support from the Department of Canadian Heritage.
5 Must-Use AI Tools to Supercharge Your Productivity



5 Must-Use AI Tools to Supercharge Your Productivitycryptouniversityoffi 5 Must-Use AI Tools to Supercharge Your Productivity!
AI is changing the game! 🚀 From research to creativity and coding, here are 5 powerful AI tools you should try.
NotebookLM
📚 NotebookLM – Your AI Research Assistant
✅ Organizes & summarizes notes
✅ Generates insights from multiple sources
✅ Ideal for students, researchers & writers
📝 Boost your productivity with smarter note-taking!
Napkin.ai
🎨 Napkin.ai – The Creativity Booster
✅ Connects and organizes ideas
✅ Perfect for writers, designers & entrepreneurs
✅ Acts as your AI-powered brainstorming partner
💡 Unleash your creativity effortlessly!
DeepSeek
🔍 DeepSeek – Smarter AI Search
✅ Delivers deeper & more precise search results
✅ Analyzes large datasets for better insights
✅ Ideal for professionals & researchers
🔎 Find what you need—faster & smarter!
ChatGPT
💬 ChatGPT – Your AI Chat Assistant
✅ Answers questions, writes content & assists in coding
✅ Helps businesses with customer support
✅ Boosts learning & productivity
🤖 From content to coding—ChatGPT does it all!
Devin AI
💻 Devin AI – AI for Coders
✅ Writes, debugs & optimizes code
✅ Assists developers at all skill levels
✅ Makes coding faster & more efficient
👨💻 Let AI be your coding partner!
🚀 AI is transforming the way we work!
Combining Lexical and Semantic Search with Milvus 2.5



Combining Lexical and Semantic Search with Milvus 2.5Zilliz In short, lexical search is a way to search your documents based on the keywords they contain, in contrast to semantic search, which compares the similarity of embeddings. We’ll be covering:
Why, when, and how should you use lexical search
What is the BM25 distance metric
How exactly does Milvus 2.5 implement lexical search
How to build an improved hybrid lexical + semantic search with Milvus 2.5
Bedrock Data Automation (Preview): Simplifying Unstructured Data Processing



Bedrock Data Automation (Preview): Simplifying Unstructured Data ProcessingZilliz Bedrock Data Automation (BDA) is a cloud-based service that simplifies the process of extracting valuable insights from unstructured content—such as documents, images, video, and audio. Come learn how BDA leverages generative AI to automate the transformation of multi-modal data into structured formats, enabling developers to build applications and automate complex workflows with greater speed and accuracy.
Revolutionizing Field Service: How LLMs Are Powering Smarter Knowledge Access...



Revolutionizing Field Service: How LLMs Are Powering Smarter Knowledge Access...Earley Information Science
Lab 4
- 1. Lab 4 Double Replacement Reactions
- 2. Reaction Types DECOMPOSITION (Lab 1) SYNTHESIS (Lab 1) COMBUSTION (Lab 1) DOUBLE REPLEACMENT (Lab 4) SINGLE REPLACEMENT (Lab 5)
- 3. Decomposition Reactions Decomposition reactions occur when a compound breaks up into the elements or in a few to simpler compounds 1 Reactant Product + Product In general: AB A + B Example: 2 H 2 O 2H 2 + O 2 Example: 2 HgO 2Hg + O 2
- 5. Synthesis reactions Synthesis reactions occur when two substances (generally elements ) combine and form a compound. (Sometimes these are called combination or addition reactions.) reactant + reactant 1 product Basically: A + B AB Example: 2H 2 + O 2 2H 2 O Example: C + O 2 CO 2
- 7. Combustion Reactions Combustion reactions occur when a hydrocarbon reacts with oxygen gas. This is also called burning!!! In order to burn something you need the 3 things in the “fire triangle”: 1) A Fuel (hydrocarbon) 2) Oxygen to burn it with 3) Something to ignite the reaction (spark)
- 8. Combustion Reactions In general: C x H y + O 2 CO 2 + H 2 O Products in combustion are ALWAYS carbon dioxide and water. (although incomplete burning does cause some by-products like carbon monoxide) Combustion is used to heat homes and run automobiles (octane, as in gasoline, is C 8 H 18 )
- 9. Combustion Reactions Edgar Allen Poe’s drooping eyes and mouth are potential signs of CO poisoning.
- 10. Procedures Various combinations of ionic compounds in solution were mixed Some of these combinations formed cloudy precipitates One of the products of these reactions was an ionic compound that was less soluble. It must have a lower Ksp.
- 11. Double Replacement Reactions Double Replacement Reactions occur when a metal replaces a metal in a compound and a nonmetal replaces a nonmetal in a compound Compound + compound product + product AB + CD AD + CB
- 12. A Precipitation Reaction NaI(aq) + Pb(NO 3 ) 2 (aq) NaNO 3 (aq) + PbI 2 (s)
- 13. NaI(aq) + Pb(NO 3 ) 2 (aq) NaNO 3 (aq) + PbI 2 (s)
- 14. NaI(aq) + Pb(NO 3 ) 2 (aq) NaNO 3 (aq) + PbI 2 (s) Balance the molecular equation above. Write the total ionic equation. Write the net ionic equation.
- 15. A demonstration Pb(NO 3 ) 2 (aq) + KI(aq) There are four different ions in the mix. Visualize the collisions.
- 16. Which collisions will result in a weak ionic bond easily broken by another collision? Which collisions will not even occur due to repulsions between the ions? Which collisions will form strong ionic bonds and clumps of ions visible as a cloudy precipitate? Pb(NO 3 ) 2 (aq) + 2 KI(aq) 2 KNO 3 (aq) + PbI 2 (s)
- 17. Visualizing the Double Replacement Reaction Ionic equations show the ions in solution AgNO 3 (aq) + NaCl(aq) NaNO 3 (aq) + AgCl(s) Ions in solution are actually dissociated or separated. In the solid the ions are together Ag + (aq) + NO 3 - (aq) + Na + (aq) + Cl - (aq) Na + (aq) + NO 3 - (aq) + AgCl(s) Notice Na + and NO 3 - ions have not changed. They are spectator ions. Net ionic equation leaves out the spectator ions. Ag + (aq) + Cl - (aq) AgCl(s)
- 18. Writing Net Ionic Equations Write the balanced molecular equation. Write the ionic equation showing the strong electrolytes completely dissociated into cations and anions. Cancel the spectator ions on both sides of the ionic equation AgNO 3 ( aq ) + NaCl ( aq ) AgCl ( s ) + NaNO 3 ( aq ) Ag + + NO 3 - + Na + + Cl - AgCl ( s ) + Na + + NO 3 - Ag + + Cl - AgCl ( s ) Write the net ionic equation for the reaction of silver nitrate with sodium chloride.
- 19. Write the balanced equation for this double replacement reaction. Which collisions will result in a weak ionic bond easily broken by another collision? Which collisions will not even occur due to repulsions between the ions? Which collisions will form strong ionic bonds and clumps of ions visible as a cloudy precipitate? Write the net ionic equation for the reaction.
- 20. Write the balanced equation for this double replacement reaction. Which collisions will result in a weak ionic bond easily broken by another collision? Which collisions will not even occur due to repulsions between the ions? Which collisions will form strong ionic bonds and clumps of ions visible as a cloudy precipitate?
- 21. Write the balanced equation for this double replacement reaction. Which collisions will result in a weak ionic bond easily broken by another collision? Which collisions will not even occur due to repulsions between the ions? Which collisions will form strong ionic bonds and clumps of ions visible as a cloudy precipitate? How is this solution at equilibrium and saturated?
- 22. Solubility Rules
- 23. Forming the green nickel (II) hydroxide precipitate Write a balanced equation that would lead to the formation of the green precipitate. What does the insolubility of nickel (II) hydroxide suggest about the value of its Ksp?
- 24. Gravimetric Analysis- A quantitative analysis of a double replacement reaction The solid produced in the reaction is isolated by filtering and rinsing. After the filter and solid are dried, the mass of the barium sulfate is measured.
- 25. Procedural questions. How does filtering separate? What goes through the filter? What remains in the filter? Why must the filter and solid be rinsed with water? What is removed in this process? Why must the filter and solid be heated to dryness? What is removed in this process?
- 26. Another double replacement reaction Mixing an acid and base
- 27. And yet another double replacement reaction Ba(OH) 2 (aq) + H 2 SO 4 (aq)
- 28. Net Ionic Equations These are the same as total ionic equations, but you should cancel out ions that appear on BOTH sides of the equation Total Ionic Equation: 2 K + + CrO 4 -2 + Pb +2 + 2 NO 3 - PbCrO 4 (s) + 2 K + + 2 NO 3 - Net Ionic Equation: CrO 4 -2 + Pb +2 PbCrO 4 (s)
- 29. Write equations (molecular, total and net ionic) for the reaction shown above. Explain the formation of the precipitate and the ions remaining in solutions by describing the collisions and attractions.
- 30. Ions in solution are dissociated attracted to the water by ion-dipole attractions. While ions in the precipitate held in fixed positions by ionic bonds between the ions H 2 O
- 31. Precipitation Reactions Precipitate – insoluble solid that separates from solution net ionic equation Na + and NO 3 - are spectator ions “ If you’re not a part of the solution, then you’re a part of the precipitate!” Pb 2+ + 2NO 3 - + 2Na + + 2I - PbI 2 ( s ) + 2Na + + 2NO 3 - Pb(NO 3 ) 2 ( aq ) + 2NaI ( aq ) PbI 2 ( s ) + 2NaNO 3 ( aq ) precipitate Pb 2+ + 2I - PbI 2 ( s )
- 32. Chemistry In Action: An Undesirable Precipitation Reaction CO 2 ( aq ) CO 2 ( g ) Ca 2+ ( aq ) + 2HCO 3 ( aq ) CaCO 3 ( s ) + CO 2 ( aq ) + H 2 O ( l ) -
- 33. Try this one! Write the molecular, total ionic, and net ionic equations for this reaction: Silver nitrate reacts with Lead (II) Chloride in hot water. Molecular: Total Ionic: Net Ionic:
- 34. Total Ionic Equations Molecular Equation: K 2 CrO 4 + Pb(NO 3 ) 2 PbCrO 4 + 2 KNO 3 Soluble Soluble Insoluble Soluble Total Ionic Equation: 2 K + + CrO 4 -2 + Pb +2 + 2 NO 3 - PbCrO 4 (s) + 2 K + + 2 NO 3 -
- 35. Solubility Table

