S I L V E R: Reaction of Silver Group Reagent Formula Color and Nature HCL
S I L V E R: Reaction of Silver Group Reagent Formula Color and Nature HCL
Uploaded by
Pharma0 ratings0% found this document useful (0 votes)
132 views1 pageThis document summarizes the reaction of silver, lead, and mercury group cations with various reagents. For silver, salts such as AgCl and AgOH are formed upon the addition of HCl and NH4OH, respectively. Lead salts produced include PbCl2, Pb(OH)2, and PbCrO4. Mercury reacts to form compounds like Hg2Cl2, HgO, and Hg2CrO4. The color and solubility of the precipitates formed in each reaction are also noted.
Original Description:
Original Title
Reaction-of-Silver-Group
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document summarizes the reaction of silver, lead, and mercury group cations with various reagents. For silver, salts such as AgCl and AgOH are formed upon the addition of HCl and NH4OH, respectively. Lead salts produced include PbCl2, Pb(OH)2, and PbCrO4. Mercury reacts to form compounds like Hg2Cl2, HgO, and Hg2CrO4. The color and solubility of the precipitates formed in each reaction are also noted.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
132 views1 pageS I L V E R: Reaction of Silver Group Reagent Formula Color and Nature HCL
S I L V E R: Reaction of Silver Group Reagent Formula Color and Nature HCL
Uploaded by
PharmaThis document summarizes the reaction of silver, lead, and mercury group cations with various reagents. For silver, salts such as AgCl and AgOH are formed upon the addition of HCl and NH4OH, respectively. Lead salts produced include PbCl2, Pb(OH)2, and PbCrO4. Mercury reacts to form compounds like Hg2Cl2, HgO, and Hg2CrO4. The color and solubility of the precipitates formed in each reaction are also noted.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 1
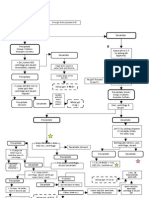
Reaction of Silver Group
Reagent Formula Color and Nature
HCL AgCl white curdy ppt to gray ppt when exposed to light
S a) boiling water insoluble
I b) Excess
Ag(NH3)2+ or Ag(NH3)2 Cl colorless solution
NH4OH
L NH4OH AgOH white ppt
V a) excess Ag(NH3)2+ or Ag(NH3)2 OH soluble- colorless solution
NaOH AgOH to Ag2O white to brown ppt
E a) excess insoluble
R K2CrO4 Ag2CrO4 brownish red ppt
Ag+ a) NaOH insouble
KI AgI canary yellow ppt
a) excess insoluble
Reagent Formula Color and Nature
HCL PbCl2 white crystal ppt
a) boiling water Aqueous Solution of PbCl2 soluble- colorless solution
L b) Excess
insoluble
NH4OH
E NH4OH Pb(OH)2 white ppt
A a) excess insoluble
NaOH Pb(OH)2 white amorphous ppt
D a) excess Pb(OH)42- or Na2PbO2 soluble-colorless solution
Pb2+ K2CrO4 PbCrO4 yellow ppt
a) NaOH Na2PbO2 soluble- yellow solution
KI PbI2 yellow ppt
a) excess insoluble
Reagent Formula Color and Nature
M HCL Hg2Cl2 white curdy ppt
a) boiling water insoluble
E b) Excess
Hg2NH2 and HgO white and black ppt
R NH4OH
NH4OH HgO.HgNH2NO3 and HgO white and black ppt
C a) excess insoluble
U NaOH Hg2O black ppt
R a) excess insoluble
K2CrO4 Hg2CrO4 orange red ppt but brownish red on boiling
Y a) NaOH insoluble
Hg22+
KI Hg2I2 greenish yellow or green ppt
a) excess Hg2I4- and HgO / K2HgI and HgO colorless solution with black ppt
You might also like
- Admission Test Exam Question Sep 2017Document3 pagesAdmission Test Exam Question Sep 2017masud77% (13)
- Lab 6-The Silver GroupDocument6 pagesLab 6-The Silver Groupsteph002100% (1)
- Cations Separation ExpDocument53 pagesCations Separation ExpDrReh E. AzoozNo ratings yet
- Pha612 Lab Expt 6 Reactions and Analysis of The Ammonium Sulfide Group PDFDocument4 pagesPha612 Lab Expt 6 Reactions and Analysis of The Ammonium Sulfide Group PDFAmmonium ChlorideNo ratings yet
- Case Studies in Anthropology Optional UpscDocument8 pagesCase Studies in Anthropology Optional UpscSaiVenkatNo ratings yet
- Experiment 6 Reaction and Analysis of Group Iii Cations Ions NH OH Excess NH OH NH CI and NH OH (NH) S Naoh Excess Naoh Na O or H O ZNDocument4 pagesExperiment 6 Reaction and Analysis of Group Iii Cations Ions NH OH Excess NH OH NH CI and NH OH (NH) S Naoh Excess Naoh Na O or H O ZNJamille SucalditoNo ratings yet
- Comparative Reactions of The Hydrogen Sulfide GroupDocument5 pagesComparative Reactions of The Hydrogen Sulfide GroupPATRICIA ROSE SORIANO100% (1)
- Comparative Reactions of The Ammonium Sulfide GroupDocument2 pagesComparative Reactions of The Ammonium Sulfide GroupPharmaNo ratings yet
- Pha612 Lab Expt 6 Reactions and Analysis of The Ammonium Sulfide Group PDFDocument4 pagesPha612 Lab Expt 6 Reactions and Analysis of The Ammonium Sulfide Group PDFAmmonium ChlorideNo ratings yet
- Pharchem LecDocument15 pagesPharchem LecNinna San Juan100% (1)
- PharmAnal 5Document22 pagesPharmAnal 5Aaron Jhulian SimbitNo ratings yet
- Bot Lab DataDocument1 pageBot Lab DataBillQueNo ratings yet
- SEATWORK 3.1 - Pharmaceutical Aids and NecessitiesDocument3 pagesSEATWORK 3.1 - Pharmaceutical Aids and NecessitiesJoseph Xerxel CabilteNo ratings yet
- Phar Chem Finals - Chapt 1-4 ExercisesDocument7 pagesPhar Chem Finals - Chapt 1-4 Exercisesjeniccax17100% (1)
- Orgmed-3 2019Document91 pagesOrgmed-3 2019Joslin Roz Galilea100% (1)
- Handout For Qualitative AnalysisDocument9 pagesHandout For Qualitative AnalysisJarvin TanNo ratings yet
- PharmChem-1 Lab Exp#01 - Analysis of Group I CationsDocument3 pagesPharmChem-1 Lab Exp#01 - Analysis of Group I CationsdavenNo ratings yet
- PHA611 LAB-Group4 Lab ReportDocument2 pagesPHA611 LAB-Group4 Lab ReportAcuCJamNo ratings yet
- UST Pharmacy Org Chem Lec MonthliesDocument72 pagesUST Pharmacy Org Chem Lec MonthliesGab ParagasNo ratings yet
- Preps 4-61Document57 pagesPreps 4-61fianaNo ratings yet
- Qualitative Analysis of CationsDocument0 pagesQualitative Analysis of CationsKaran SaxenaNo ratings yet
- Chem 41 Lab Formal Report 01 - Preparation of Buffers & Amino Acids As AmpholytesDocument13 pagesChem 41 Lab Formal Report 01 - Preparation of Buffers & Amino Acids As AmpholytesFaith VillahermosaNo ratings yet
- PharmChem-1 Lab Exp#02 - Analysis of Group II CationsDocument2 pagesPharmChem-1 Lab Exp#02 - Analysis of Group II CationsdavenNo ratings yet
- Chap 04 Acids and BasesDocument22 pagesChap 04 Acids and BasesavNo ratings yet
- INORG LAB Reactions of Alkali GroupDocument1 pageINORG LAB Reactions of Alkali GroupGrace HernandezNo ratings yet
- Weak Acid Base NotesDocument49 pagesWeak Acid Base NotesJankel L PahuyoNo ratings yet
- Chem 18.1 Experiment 9 Qualitative Analysis - Separation and Identification of CationsDocument3 pagesChem 18.1 Experiment 9 Qualitative Analysis - Separation and Identification of Cationscarmina_guerreroNo ratings yet
- Ointments: DefinitionDocument7 pagesOintments: DefinitionAnonymous XuiUo2ThNo ratings yet
- 1-History, Evolution and Milestone in PharmacyDocument29 pages1-History, Evolution and Milestone in PharmacyEliza Quines QuizonNo ratings yet
- 1 Introduction To Pharmaceutical Dosage Forms Part1Document32 pages1 Introduction To Pharmaceutical Dosage Forms Part1Joanna Carla Marmonejo Estorninos-Walker100% (1)
- University of Santo TomasDocument5 pagesUniversity of Santo TomasJanine MontaNo ratings yet
- 5 With Notes PDFDocument2 pages5 With Notes PDFKimberley Anne SeeNo ratings yet
- Solubility and Simultaneous Equilibria: Chemistry: The Molecular Nature of Matter, 6EDocument59 pagesSolubility and Simultaneous Equilibria: Chemistry: The Molecular Nature of Matter, 6EEriani WulandariNo ratings yet
- PHAR3 LAB-Syrups&Mucilage RVDocument3 pagesPHAR3 LAB-Syrups&Mucilage RVAbigail Beatrice LumbaoNo ratings yet
- Inorg Med.1.1-1Document38 pagesInorg Med.1.1-1Kathleen Joy ArutaNo ratings yet
- HtwoO and BufferDocument7 pagesHtwoO and BufferManila MedNo ratings yet
- 02.plant StructureDocument21 pages02.plant StructureAnonymous 5698Hl5ZxNo ratings yet
- Perspective in Pharmacy Lesson 1Document10 pagesPerspective in Pharmacy Lesson 1Adya AeshaNo ratings yet
- Pharm 222L - Activity 1 - Group5Document9 pagesPharm 222L - Activity 1 - Group5france hambon100% (1)
- Investigational New Drug Application (IND)Document27 pagesInvestigational New Drug Application (IND)Pharmacology MnemonicsNo ratings yet
- Acid Base Titrations 11II PDFDocument35 pagesAcid Base Titrations 11II PDFŠĭlệncěIšmyPŕIdệNo ratings yet
- Chapter 2 Pharmaceutical Aids and NecessitiesDocument11 pagesChapter 2 Pharmaceutical Aids and NecessitiesZarah Pauline Jimenez100% (2)
- Types of Titrimetric AnalysisDocument62 pagesTypes of Titrimetric AnalysisJacqueline BaquiranNo ratings yet
- Pharmaceutical Aids and Necessities (Report Pharchem 1-Group 1)Document109 pagesPharmaceutical Aids and Necessities (Report Pharchem 1-Group 1)NicoleTrishiaDeparineNo ratings yet
- Plant Leaves: Pha 611 Lec Pharmaceutical Botany With TaxonomyDocument44 pagesPlant Leaves: Pha 611 Lec Pharmaceutical Botany With TaxonomyLlang LleavNo ratings yet
- Exercises Student EdDocument10 pagesExercises Student EdKukkiboNo ratings yet
- 3.2 Dispensing AdrDocument5 pages3.2 Dispensing Adrslu.laza.joeannNo ratings yet
- Uptake of Carbon Dioxide and Evolution of OxygenDocument5 pagesUptake of Carbon Dioxide and Evolution of OxygenBeatrice Lianne Francisco EstacioNo ratings yet
- Lab Report Chemical EquilibriumDocument5 pagesLab Report Chemical EquilibriumMingNo ratings yet
- Pharmacognosy PPT 2ND ShiftDocument105 pagesPharmacognosy PPT 2ND ShiftJohn TecsonNo ratings yet
- Effectiveness of Insulin Plant (Costus Igneus) Leaves As Tea On Lowering Blood Sugar LevelDocument43 pagesEffectiveness of Insulin Plant (Costus Igneus) Leaves As Tea On Lowering Blood Sugar Levelcharles estradaNo ratings yet
- Reaction Stoichiometry Part 2 and Solution Stoichiometry PDFDocument10 pagesReaction Stoichiometry Part 2 and Solution Stoichiometry PDFGeraldNo ratings yet
- Group 1A - Alkali Metals Elements Compounds Pharmaceutical Uses Chemical Name Chemical FormulaDocument2 pagesGroup 1A - Alkali Metals Elements Compounds Pharmaceutical Uses Chemical Name Chemical FormulaCharlyn Keith100% (1)
- Group IV Cations Anions FlowchartsDocument2 pagesGroup IV Cations Anions FlowchartsFaith DomingoNo ratings yet
- Group 1 and 4 Cation AnalysisDocument26 pagesGroup 1 and 4 Cation Analysistwinkledreampoppies100% (1)
- Phar Chem 1 OBTLDocument6 pagesPhar Chem 1 OBTLtallulaNo ratings yet
- Qualitative Test For Elements in Organic CompoundsDocument4 pagesQualitative Test For Elements in Organic CompoundsFlorence Lynn BaisacNo ratings yet
- BS Pharmacy - ProspectusDocument9 pagesBS Pharmacy - ProspectusDomz BucadNo ratings yet
- Mariano Marcos State University: Pharmaceutical Botany With TaxonomyDocument7 pagesMariano Marcos State University: Pharmaceutical Botany With TaxonomyKaizenNo ratings yet
- Acid and Base TheoryDocument25 pagesAcid and Base TheoryPtrick MahnyNo ratings yet
- Major Intra and Extracellular IonsDocument29 pagesMajor Intra and Extracellular IonsRasel IslamNo ratings yet
- Expt 4Document1 pageExpt 4Mia MistypuffNo ratings yet
- Xii Physics Lab Manual Sec BDocument29 pagesXii Physics Lab Manual Sec BB. AsmaNo ratings yet
- Understanding Power FlowDocument20 pagesUnderstanding Power FlowelhaffarNo ratings yet
- POCO X2 Review PDFDocument10 pagesPOCO X2 Review PDFASHISH KUMAR MISRANo ratings yet
- Fluid Mechanics Problem 1: Pressures Are Sometimes Determined by Measuring The Height of A Column ofDocument21 pagesFluid Mechanics Problem 1: Pressures Are Sometimes Determined by Measuring The Height of A Column ofEngineering NepalNo ratings yet
- Споменик српске краљевске академије XVDocument76 pagesСпоменик српске краљевске академије XVIgorьNo ratings yet
- QITP 002 Part 1Document12 pagesQITP 002 Part 1ivanbfNo ratings yet
- Watu Waingapu D Umbu Mehang Kunda 3Document7 pagesWatu Waingapu D Umbu Mehang Kunda 3agusNo ratings yet
- Pantone PDFDocument53 pagesPantone PDF2dlmediaNo ratings yet
- Ingles para Negocios (Tema 01)Document31 pagesIngles para Negocios (Tema 01)Jota ErreNo ratings yet
- 1.2.1 Typical Sections: Precast-Pretensioned Concrete Girder BridgesDocument12 pages1.2.1 Typical Sections: Precast-Pretensioned Concrete Girder Bridgesmohamed ahmedNo ratings yet
- Nature of Advertising WritingDocument12 pagesNature of Advertising WritingNkoli OgboluNo ratings yet
- 23 - Braga Filofteia, Diaconescu FlorinDocument6 pages23 - Braga Filofteia, Diaconescu FlorinAda KuprowskaNo ratings yet
- TM Smaw NC IiDocument137 pagesTM Smaw NC IiSam Louis Lepiten100% (2)
- Iap Sms Textbook of Community MedicineDocument2 pagesIap Sms Textbook of Community MedicineIndhumathi0% (1)
- Aquatrol: An Android App For Aquaponics Control and Monitoring System Utilizing IotDocument5 pagesAquatrol: An Android App For Aquaponics Control and Monitoring System Utilizing IotRAZOUL BOQUIRONNo ratings yet
- Project PDFDocument62 pagesProject PDFAnonymous BPYuxkRaSLNo ratings yet
- Ni P F Year Per I Year N IDocument5 pagesNi P F Year Per I Year N IRyan CalicaNo ratings yet
- Multiphase Reactors: CPE624 Faculty of Chemical EngineeringDocument38 pagesMultiphase Reactors: CPE624 Faculty of Chemical EngineeringUzuki ADNo ratings yet
- Skeletal System Lab ReportDocument5 pagesSkeletal System Lab Reportapi-296603950100% (1)
- Discreate Mathmatics: Tutorial Name: College Id: BranchDocument14 pagesDiscreate Mathmatics: Tutorial Name: College Id: BranchMohdNo ratings yet
- Hydraulics and Floodplain Modeling - HY-8 Modeling Wizard: WMS 8.4 TutorialDocument11 pagesHydraulics and Floodplain Modeling - HY-8 Modeling Wizard: WMS 8.4 TutorialAlireza MohebzadehNo ratings yet
- Prepositions of Place and Direction PDFDocument3 pagesPrepositions of Place and Direction PDFTan DanNo ratings yet
- TCP Financial Analysis Q1Document7 pagesTCP Financial Analysis Q1Dulakshi RanadeeraNo ratings yet
- 2013 Matrices Multiple Choice RevisionDocument22 pages2013 Matrices Multiple Choice Revisionbanking kida all hereNo ratings yet
- Concrete Dams Case Histories of Failures and Nonfailures With Back CalculationsDocument100 pagesConcrete Dams Case Histories of Failures and Nonfailures With Back Calculationswendydy6No ratings yet
- ASTM B505-B505M-12 Standard Specification For Cooper Alloy Continuous CastingsDocument10 pagesASTM B505-B505M-12 Standard Specification For Cooper Alloy Continuous CastingsAarón Escorza MistránNo ratings yet
- MCQ of DC Machines Madhuri NewDocument31 pagesMCQ of DC Machines Madhuri NewAnkit KumarNo ratings yet
- Change ManagementDocument41 pagesChange ManagementuzaimyNo ratings yet