Course Corriculum
Course Corriculum
Uploaded by
Akhil KumarCopyright:
Available Formats
Course Corriculum
Course Corriculum
Uploaded by
Akhil KumarCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Copyright:
Available Formats
Course Corriculum
Course Corriculum
Uploaded by
Akhil KumarCopyright:
Available Formats
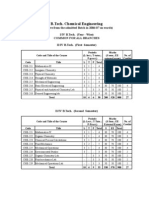
Course Curriculum
(Course Structure and Syllabi)
for
Bachelor of Technology
in
Chemical Engineering
(Second Year Onwards)
Department of Chemical Engineering
National Institute of Technology Hamirpur
Hamirpur – 177 005 (India)
Second Year
3rd Semester 4th Semester
SN Code Subject L T P Credits SN Code Subject L T P Credits
Engineering
1 HS-203 Organizational Behaviour 3 0 0 3 1 MA-203 3 1 0 4
Mathematics-III
2 CH-211 Fluid Mechanics 3 1 0 4 2 CH-221 Heat Transfer 3 1 0 4
Chemical Engineering Chemical Engineering
3 CH-212 3 1 0 4 3 CH-222 3 1 0 4
Thermodynamics-I Thermodynamics-II
Chemical Process Industrial Pollution
4 CH-213 3 1 0 4 4 CH-223 3 1 0 4
Calculations Abatement
5 CH-214 Mechanical Operation 3 1 0 4 5 CH-224 Chemical Technology 3 0 0 3
6 CH-215 Fluid Mechanics Lab 0 0 2 1 6 CH-225 Computational Lab 0 0 2 1
7 CH-216 Thermodynamics Lab 0 0 2 1 7 CH-226 Heat Transfer Lab 0 0 2 1
Mechanical Operation Chemical Technology
8 CH-217 0 0 2 1 8 CH-227 0 0 2 1
Lab Lab
Total Hours = 25 22 Total Hours = 25 22
Third Year
5th Semester 6th Semester
SN Code Subject L T P Credits SN Code Subject L T P Credits
1 CH-311 Mass Transfer-I 3 1 0 4 1 CH-321 Mass Transfer-II 3 1 0 4
Chemical Reaction Chemical Reaction
2 CH-312 3 1 0 4 2 CH-322 3 1 0 4
Engineering-I Engineering-II
Process Equipment Process Equipment
3 CH-313 3 0 0 3 3 CH-323 3 1 0 4
Design-I Design-II
Process Dynamics and Process Modeling and
4 CH-314 3 1 0 4 4 CH-324 3 0 0 3
Control Simulation
5 OET Open Elective-I 3 0 0 3 5 OET Open Elective-II 3 0 0 3
Chemical Reaction
6 CH-315 0 0 2 1 6 CH-325 Mass Transfer Lab 0 0 2 1
Engineering Lab
Process Dynamics and
7 CH-316 0 0 2 1 7 CH-326 Process Simulation Lab 0 0 2 1
Control Lab
Industrial Pollution
8 CH-317 0 0 2 1 8 CH-327 Seminar 0 0 2 1
Abatement Lab
Total Hours = 24 21 Total Hours = 24 21
Department of Chemical Engineering, NIT Hamirpur Page 2 of 56
Fourth Year
7th Semester 8th Semester
SN Code Subject L T P Credits SN Code Subject L T P Credits
Process Plant Design Engineering Economics
1 CH-411 3 0 0 3 1 HS-404 3 0 0 3
and Economics and Accountancy
Petroleum Refining
Industrial Safety and
2 CH-412 and Petrochemical 3 0 0 3 2 CH-421 3 0 0 3
Hazard Management
Engineering
3 DET Professional Elective-I 3 0 0 3 3 DET Professional Elective-III 3 0 0 3
4 DET Professional Elective-II 3 0 0 3 4 DET Professional Elective-IV 3 0 0 3
Industrial Training
5 CH-413 0 0 2 1 5 CH-422 General Proficiency 0 0 0 1
Presentation
Major Project Major Project
6 CH-414 0 0 12 6 6 CH-423 0 0 12 6
(Stage–I) (Stage–II)
Total Hours = 26 19 Total Hours = 24 19
Semester Wise Credits
Semester
1st 2nd 3rd 4th 5th 6th 7th 8th Total
Credits
24 24 22 22 21 21 19 19 172
Hours/week
28 28 25 25 24 24 26 24 204
Department of Chemical Engineering, NIT Hamirpur Page 3 of 56
Professional Elective Courses
Professional Elective – I
CH-430 Optimization of Chemical Processes
CH-431 Computational Fluid Dynamics
CH-432 Instrumental Analytical Techniques
CH-433 Soft Computing Methods in Chemical Engineering
Professional Elective-II
CH-450 Fuel Cells and Hydrogen Energy
CH-451 Energy Engineering
CH-452 Solid Waste Management
CH-453 Reservoir Engineering
Professional Elective-III
CH-440 Biochemical Engineering
CH-441 Food Science and Engineering
CH-442 Fertilizer Technology
CH-443 Novel Separation Processes
Professional Elective-IV
CH-460 Polymer Science and Engineering
CH-461 Colloid and Interface Science
CH-462 Nanomaterials and Nanofabrication
CH-463 Heterogeneous Catalysis and Catalytic Processes
Open Elective Courses
Open Elective-I
CH-306 Energy and Environmental Engineering
CH-370 Nanoscience and Nanotechnology
Open Elective-II
CH-306 Energy and Environmental Engineering
CH-380 Industrial Safety and Hazard Management
Department of Chemical Engineering, NIT Hamirpur Page 4 of 56
Course Name: Organizational Behaviour
Course Code: HS-203
Course Type: Core
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To impart knowledge about the behavioural aspects related to professional organizations
To introduce the fundamental concepts relevant to understanding of individual & group behavior in the organization
To enable the students to understand the applied organizational themes like perception, motivation, interpersonal
relationships, group dhynamics, leadership theories, role of power & politices in organizational context, conflict and
negotiation, organizational diversity, dynamics of personality, attitude and job satisfaction, etc.
Unit Number Course Content Lectures
UNIT-01 Organizational Behavior (OB): Concept, nature, characteristics, conceptual 04L
foundations, determinants and importance, management functions, role & skills,
disciplines that contribute to the field of OB, Challenges & Opportunities for OB,
diversity in Organizations, attitudes & Job satisfaction.
UNIT-02 Perception: Concept, nature, process, importance, management and behavioral 08L
applications of perception. Personality: concept, nature, types and theories of
personality shaping. Learning; concept and theories of learning.
UNIT-03 Motivation: concept, principles, theories-content, process & contemporary, Monetary 06L
and non-monetary motivation, applications of motivation. Leadership: Concept,
functions, styles, and theories of leadership- trait, behavioural, and situational.
UNIT-04 Group and Interpersonal Relationship: Analysis of Interpersonal Relationship, 05L
developing interpersonal relationship, Group Dynamic: Definition of Group, stages of
Group Development, Punctuated Equilibrium Model, Group Structure, Group Decision
Making, understanding work teams.
UNIT-05 Organizational Power and Politics: concept of power, structure of power, 06L
classification of power, contrasting leadership & power, dependence a key to power,
causes & consequences of political behaviour. Organizational conflict: view of conflict,
conflict process, negotiation & bargaining strategies.
UNIT-06 Conflict and Negotiation: conflict definition in conflict thought: Traditional view, the 07L
Human relation view, interactionist view. Functional versus dysfunctional conflict,
conflict process. Negotiation Bargaining strategies, the negotiation process and issues
in negotiation.
Course Outcomes
Upon successful completion of the course, the students will be able to
CO1: Identify the challenges of the present organization
CO2: Describe the organizational system
CO3: Apply the principles of organizational behavior to inculcate the habit of team work and which is essential for the
organization
CO4: Assess the role of psychological and social principal in improvement of efficiency as well as quality of empoyee life
Books and References
1. Organizational Behavior by Robbins, S.P., Prentice Hall of India.
2. Organizational Behavior by Luthans F., McGraw-Hill.
3. Human Behavior at Work: Organizational Behavior by Davis K., Tata McGraw-Hill.
Department of Chemical Engineering, NIT Hamirpur Page 5 of 56
Course Name: Fluid Mechanics
Course Code: CH-211
Course Type: Core
Contact Hours/Week: 3L+ 1T Course Credits: 04
Course Objectives
To understand basic concepts of fluid flow and their application in solving engineering problems.
To teach fundamental concepts in fluid mechanics and apply them to real problems.
To develop and use momentum and energy conservations laws and Bernoulli’s equation.
To explain basics behind various measurements, pipe fitting, valves, pump types and centrifugal pump.
Unit Number Course Content Lectures
Introduction: Ideal and real fluids, specific weight, mass density and specific gravity,
viscosity and its measurements, pressure and temperature dependence of viscosity, 06L
UNIT-01
surface tension and capillarity, Newtonian and non-Newtonian fluids, dimensional
analysis.
Fluids Static: Pressure, hydrostatics law, Pascal’s law, manometers and pressure
UNIT-02 measurement, forces on inclined plane and curved submerged surfaces. 04L
Fluids Kinematics and Dynamics: Classification of fluid flows, Eulerian and Lagrangian
approach, substantial derivative, laminar and turbulent flow, Stream function, potential
function, vortex flow (free and forced). Continuity equation, Navier-Stoke’s equation,
UNIT-03 11L
Bernoulli’s equation and its application, correction factors, energy and hydraulic grade
lines. flow and velocity measurement devices: Pitot tube, hot wire anemometer, Vena-
contracta, notches and weirs, orificemeter, venturimeter, rotameter.
Incompressible Viscous Flow: General characteristics of pipe flow-laminar, turbulent,
entrance region, fully developed flow. Hagen-Poiseuille equation, shear stress
UNIT-04 distribution and velocity profiles, major and minor losses in pipes, fittings, noncircular 08L
ducts, friction factor, pipe roughness, Moody chart,Boundary layer theory,drag force, lift
and drag coefficients, drag on flat plate, circular cylinder and sphere.
Pumps and compressors: Classification and working of pumps: centrifugal,
reciprocating, piston, plunger, gear and diaphragm pumps, Work and power input,
UNIT-05 cavitation, NPSH, maximum suction lift, specific and minimum speed, pump losses and 07L
efficiencies,Multistage pumps,fans, blowers and compressors.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Apply basic concepts of fluid mechanics in manometers, flow and velocity measurements.
CO2: Develop dimensionless groups and describe turbulent flow.
CO3: Apply principles of conservation of mass, momentum and energy and Bernoulli’s equation.
CO4: Calculate losses in pipes and fittings and do basic calculations on pumps.
Books and References
1. Unit Operations of Chemical Engineering by J.C. Smith, W.L. McCabe, and P.H. Harriot, McGraw-Hill.
2. Fluid Mechanics and Fluid Power Engineering by D.S. Kumar, S. K. Kataria and Sons.
3. Fluid Mechanics by F.M. White, McGraw-Hill.
4. Fluid Mechanics: Fundamentals and Applications by J.M. Cimbala, and Y.A. Cengel, McGraw-Hill.
5. Hydraulics and Fluid Mechanics by P.N. Modi, and S.M. Seth, Delhi Standard Publishers.
Department of Chemical Engineering, NIT Hamirpur Page 6 of 56
Course Name: Chemical Engineering Thermodynamics-I
Course Code: CH-212
Course Type: Core
Contact Hours/Week: 3L+ 1T Course Credits: 04
Course Objectives
To impart knowledge about the basic concepts of chemical engineering thermodynamics.
To introduce the fundamental concepts relevant to different chemical process.
To apprise students of various laws of thermodynamics and their applications.
Unit Number Course Content Lectures
Fundamental Concepts and Definitions: Closed, open and isolated system, intensive
and extensive properties, path and state functions, reversible and irreversible process,
UNIT-01 06L
zeroth and first laws of thermodynamics, internal energy, enthalpy, heat capacity, heat
and work, steady state energy, applications.
P-V-T Behaviour of Pure Substances: Ideal gases, equations of state, Van der Waals,
Redlich-Kwong and Virial equations, principle of corresponding states, critical and
UNIT-02 07L
pseudo critical properties, compressibility charts, steam table, generalized correlations
for gases and liquids.
Second Law of Thermodynamics: Limitations of first law, general statements of second
law, concept of entropy, calculation of entropy changes, Carnot’s principle, absolute scale
UNIT-03 08L
of temperature, Clausius inequality, entropy and irreversibility, statistical explanation of
entropy, third law of thermodynamics, available energy and exergy.
Heat Effects in Chemical Reactions: Standard heat of formation, combustion and
UNIT-04 reaction, effect of temperature on heat of reaction, temperature of reactions, adiabatic 05L
reaction temperature.
Refrigeration and Liquefaction: COP, vapour compression cycles, Carnot cycle, air
UNIT-05 compression, general properties of refrigerant, choice of refrigerant, absorption 06L
refrigeration, heat pump, Joule-Thomson expansion and liquefaction processes.
Power Cycles: Rankine cycle, internal combustion engine cycles, gas-turbine power
UNIT-06 04L
plant cycle.
Course Outcomes
Upon successful completion of the course, the students will be able to
CO1: Identify and calculate the thermodynamic properties of pure substances.
CO2: Describe the different thermodynamic aspects based on fundamental concepts.
CO3: Apply principle of thermodynamics for analysis of various processes.
CO4: Assess the importance of applications of thermodynamic laws in related fields.
Books and References
1. Chemical Engineering Thermodynamics by Y.V.C. Rao, Universities Press, Hyderabad, 1997.
2. Introduction to Chemical Engineering Thermodynamics by J.M. Smith, H.C. VanNess, and M.M. Abbott, Tata
McGraw Hill, 2010.
3. Chemical and Process Thermodynamics by B.G. Kyle, Prentice Hall PTR, New Jersey, 1999.
4. A Text book of Chemical Engineering Thermodynamics by K.V. Narayanan, Prentice Hall, 2013.
5. Thermodynamics: An Engineering Approach by Y.A. Cengel, and M.A. Boles, Tata McGraw-Hill, 2008.
Department of Chemical Engineering, NIT Hamirpur Page 7 of 56
Course Name: Chemical Process Calculations
Course Code: CH-213
Course Type: Core
Contact Hours/Week: 3L+1T Course Credits: 04
Course Objectives
To understand the basic units of different parameters used in various chemical process and their conversions.
To understand various physical properties and their behavior with the process conditions.
To enable students to formulate and solve material and energy balances for chemical processes.
To understand the fuels and combustion calculation, proximate and ultimate analysis.
Unit Number Course Content Lectures
Stoichiometry: Units and dimensions, stochiometric principles, composition relations,
UNIT-01 04L
density and specific gravity.
Ideal Gases and Vapor Pressure: Behaviour of ideal gases, application of ideal gas
law, volume changes with change in composition of gaseous mixtures, effect of
UNIT-02 06L
temperature on vapor pressure, vapor pressure plots vapor pressure of immiscible
liquids-solutions.
Humidity and Solubility: Humidity, relative humidity, saturation, condensation, wet
UNIT-03 06L
and dry bulb thermometry, solubility and crystallization, dissolution, solubility of gases.
Material Balance: Material balances for systems with and without chemical reactions,
UNIT-04 07L
species and elemental balance, analysis of systems with by-pass, recycle and purge.
Energy Balance: Steady state energy balance for systems with and without
chemical reactions, heat capacity of gases, liquids and solutions, heat of fusion and
UNIT-05 vaporization, calculations and application of heat of reaction and heat of formation, 07L
combustion, enthalpy-concentration charts, combustion of solids, liquids and gaseous
fuels.
Simultaneous Balances: Problems related to simultaneous steady state energy and
UNIT-06 material balance, unsteady and material balance, simultaneous material and energy 06L
balance.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Describe and solve material and energy balances simultaneously of a given process.
CO2: Calculate the bubble point and dew points of multicomponent mixtures.
CO3: Develop the skills to understand the use of psychrometric charts and determine the properties of air required in
solving vaporization and condensation problems.
CO4: Illustrate the pressure-volume-temperature relation of ideal and real gases.
Books and References
1. Basic Principles and Calculations in Chemical Engineering by D.M. Himmelblau, and J.B. Riggs, 8th edition,
Prentice Hall India, 2014.
2. Elementary Principles of Chemical Processes by R.M. Felder, R.W. Rousseau, and L.G. Bullard, 4th edition,
John Wiley& Sons, 2011.
3. Chemical Process Principles (Part-I): Material and Energy Balances by O.A. Haugen, K.M. Watson, and R.A.
Ragatz, 2nd edition, John Wiley& Sons, 2004.
4. Stoichiometry by B.I. Bhatt, and S.B. Thakore, 5th edition, McGraw Hill, 2017.
Department of Chemical Engineering, NIT Hamirpur Page 8 of 56
Course Name: Mechanical Operation
Course Code: CH-214
Course Type: Core
Contact Hours/Week: 3L+1T Course Credits: 04
Course Objectives
To impart knowledge about various operations carried out on solids in chemical industries
To introduce the fundamental of various aspects of solid handling and fluid-solid interaction
To enable the student to understand working principles of various industrial operations viz. clarification,
thickening, sedimentation, handling and storage of solid materials
Unit Number Course Content Lectures
Size Reduction and Screening: Particle size and shape, particle mass, size and
UNIT-01 shape distributions, measurement and analysis, concept of average diameter, size
reduction, crushing, grinding and law of grindings, screening equipment, capacity and 10L
effectiveness of screen, effect of mesh size on capacity of screen
Settling: Flow around a single particle, drag force and drag coefficient, settling velocity
UNIT-02 of particles in a fluid, hindered and free settling of particles, gravity sedimentation, 06L
thickening and clarification, flotation, magnetic separation
Filtration: Classification of filters, various types of cake filters, principle of cake
UNIT-03 06L
filtration, clarification filters, liquid clarification, centrifugal settling process
Agitation and Mixing: Agitation of liquids, axial flow impellers, radial flow impellers,
UNIT-04 03L
velocity and power consumption of agitated vessels, blending & mixing
Fluidization: Packed beds, bed porosity, flow through a bed of particles, fluidization &
UNIT-05 06L
fluidized bed, conditions for fluidization minimum velocity, types of fluidization
Solid Handling: Flow of solid by gravity, transport of solids by screw /belt conveyers,
UNIT-06 05L
cyclones, bag filters, electrostatic precipitators, particulate collection system
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Identify the key problems associated with operation involving fluid-solid interaction
CO2: Describe various types of equipment used in size reduction, screening, solid-solid and fluid-solid separation,
transportation of solids etc.
CO3: Apply principles of fluidization, surface energy, drag forces and other forces for solid feed processing in chemical
industries
Books and References
1. Unit Operations of Chemical Engineering by J.C. Smith, W.L. Mccabe, and P.H. Harriot, McGraw Hill, 2001.
2. Mechanical Operation for Chemical Engineers by B.C. Bhattacharya, and C.M. Narayanan, Khanna Publishers,
1990.
3. Perry’s Handbook of Chemical Engineering by D.W. Green, and R.H. Perry, McGraw Hill, 1997.
4. Unit Operations by G.G. Brown, CBS Publisher, 2004.
5. Chemical Engineering by J.M. Coulson, J.F. Richardson, and R.K. Sinnott, Vol.-2, Butterworth-Heinemann, 2003.
Department of Chemical Engineering, NIT Hamirpur Page 9 of 56
Course Name: Fluid Mechanics Lab
Course Code: CH-215
Contact Hours/Week: 2P Course Credits: 01
Course Objectives
To measure velocity, pressure and friction loss in pipe
To determine efficiency of various pumps
To calibrate various flowmeters and verify Bernoulli’s theorem
To study flow characteristic visually in a pipe and around an obstacle
List of Experiments
1. To verify Bernoulli’s equation experimentally.
2. To study the velocity distribution in a pipe and to compute the discharge by integrating the velocity profile.
3. To visualize different flow conditions and obtain the Reynolds number.
4. To calibrate Venturimeter, Orificemeter and Rotameter.
5. To find the friction factor in pipes of different diameters.
6. To determine the minor head loss coefficient for different pipe fittings.
7. To draw flow net for irrotational flow past a cylinder (or any other geometry) using Hale –Shaw apparatus.
8. To draw the characteristics curve of reciprocating pump and determine its efficiency.
9. To draw the characteristics curve of gear pump and determine its efficiency.
10. To draw the characteristics curve of jet pump and to determine its efficiency.
11. To draw characteristic curve of a centrifugal pump and determine its efficiency.
12. To study the pressure measurement
13. To estimate the kinematic viscosity using Redwood viscometer.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Perform various measurements on pressure, velocity, losses in pipes and fittings.
CO2: Select a flow meter for design purpose.
CO3: Calculate power requirements and efficiency of various types of pumps.
Department of Chemical Engineering, NIT Hamirpur Page 10 of 56
Course Name: Thermodynamics Lab
Course Code: CH-216
Contact Hours/Week: 2P Course Credits: 01
Course Objectives
To define the fundamental concepts to students in the area of thermodynamics and its applications.
To recognize the practical significance of various parameters those are involved in different fundamental
equations.
To apply the knowledge of thermodynamics in an effective manner for different applications.
List of Experiments
1. To determine the enthalpy of combustion by using Bomb calorimeter
2. To determine the melting point of liquid and solid substance by using melting point apparatus
3. To determine the vapor pressure of water at high temperature by using vapor pressure measurement apparatus
4. To determine the activity coefficient of a substance by using activity coefficient measurement apparatus
5. To study the vapor-liquid equilibria (VLE) of two phase system
6. To study the calorimetry of solid and liquid in vacuum by using adiabatic calorimeter
7. To Investigate the relationships between pressure and volume (Boyle's law) by using ideal gas law apparatus
8. To measure the dryness factor of steam by using separating & throttling calorimeter
9. To calculate the volumetric efficiency by using single stage air compressor test rig
10. To calculate the volumetric efficiency by using two stage air compressor test rig
11. To determine the thermal conductivity and thermal diffusivity of liquid and solid substances by using thermal
conductivity and thermal diffusivity measurement apparatus
12. To study the behavior and expansion processes of a perfect gas by using bench-top apparatus
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Identify practically melting point of different fluids and substances.
CO2: Describe activity coefficient, ideal gas law and adiabatic conditions.
CO3: Apply thermodynamics concepts towards various equipment and measure thermal conductivity and thermal
diffusivity.
CO4: Study various types of calorimeters, air compressors, and their efficiencies.
Department of Chemical Engineering, NIT Hamirpur Page 11 of 56
Course Name: Mechanical Operation Lab
Course Code: CH-217
Contact Hours/Week: 2P Course Credits: 01
Course Objectives
To understand the importance of various mechanical operations used in process industry.
To apply principles of basic sciences and chemical engineering for designing various size reduction,
separation and conveying equipment.
List of Experiments
1. To determinethe power consumption and study of agitation and mixing characteristic of a fluid.
2. To determinethe drag coefficient using falling ball method.
3. To determinethe collection efficiency of a cyclone separator.
4. To determinethe screening efficiency in a vibrating screen.
5. To determinethe cake and filter medium resistance of plate and frame filter press.
6. To determine the specific cake resistance in constant pressure vacuum filter.
7. To determinethe crushing efficiency of a roll crusher.
8. To determine the energy required for grinding and critical speed of ball mill.
9. To determinethe settling characteristics of a batch settler.
10. To determine the separation efficiency using trommel screen.
11. To study the screw conveyor for transport applications.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Apply the principles of unit operations through experimentation for separating solids from fluids
CO2: Demonstrate the ability to understand the various equipment used in chemical and allied industry.
Department of Chemical Engineering, NIT Hamirpur Page 12 of 56
Course Name: Engineering Mathematics-III
Course Code: MA-203
Course Type: Core
Contact Hours/Week: 3L + 1T Course Credits: 04
Course Objectives
To introduce the fundamental concepts relevant to function of complex variable, numerical differentiation and integration
and numerical solution of linear, non-linear and system of equations.
To have the idea of evaluation of real integrals using complex variable.
To understand the concept of approximating & interpolating polynomials and finding values of function at arbitrary point.
To impart knowledge of various numerical technique to solve ODE.
Unit Number Course Content Lectures
Functions of Complex Variable
Applications of De Moivre’s theorem, Exponential, Circular, Hyperbolic and Logarithmic functions
of a complex variable, Inverse Hyperbolic functions, Real and imaginary parts of Circular and
Hyperbolic functions, Summation of the series-‘C+iS’ method.
UNIT-01 12 L
Limit and derivative of complex functions, Cauchy-Riemann equations, Analytic functions and its
applications, Complex integration, Cauchy’s theorem, Cauchy’s integral formula, Series of
complex function, Taylor series, singularities and Laurent’s series, Cauchy’s residue theorem and
its application for the evaluation of real definite integrals.
Interpolation
Least square curve fit and trigonometric approximations, Finite differences and difference
UNIT-02 06L
operators, Newton’s interpolation formulae, Gauss forward and backward formulae, Sterling and
Bessel's formulae, Lagrange's interpolation.
Numerical Integration
UNIT-03 Integration by trapezoidal and Simpson’s rules 1/3 and 3/8 rule, Romberg integration, and 05L
Gaussian quadrature rule, Numerical integration of function of two variables.
Numerical Solution of Ordinary Differential Equations
Taylor series method, Picard’s method, Euler’s method, Modified Euler’s method, Runge‐Kutta
UNIT-04 07L
method. Predictor corrector methods, Adam Bashforth and Milnes method, convergence criteria,
Finite difference method.
Numerical Solution of Linear and Non Linear Equations
Non Linear Equations: Bisection Method, RegulaFalsi Method, Newton-Raphson Method, Iteration
UNIT-05 06 L
method.
Linear Equations: Jacobi and Gauss Seidel Iteration methods, Relaxation method.
Course Outcomes
Upon successful completion of the course, the student will be able to:
CO1: Understand and analyze the concept of Numerical Solution of Linear and Non Linear Equations, Ordinary Differential
Equations and Function of complex variable.
CO2: Identify an appropriate technique to solve the linear, non-linear equations, ordinary differential equations.
CO3: Formulate the problems on related topics and solve analytically.
CO4: Apply the concepts of linear, non-linear equations, differential equations and complex analysis in various engineering
problems.
CO5: Demonstrate the concepts through examples and applications.
Books and References
1. Complex Variables and Applications by R.V. Churchill, J.W. Brown,and R.F. Verhey, McGraw Hill.
2. A First Course in Complex Analysis with Applications by D.G. Zill, and P.D. Shanahan, Jones and Bartlett.
3. Numerical Methods for Scientific and Engineering Computation by M.K. Jain, S.R.K. Iyenger, and R.K. Jain, New Age
International Publishers, New Delhi.
4. Numerical Methods for Engineers and Scientists by J.D. Hoffman, 2nd edition, CRC Press.
5. Numerical Analysis Mathematics and Scientific Computing by D. Kincaid, and W. Cheney, 3rd edition, American
Mathematical Society.
Department of Chemical Engineering, NIT Hamirpur Page 13 of 56
Course Name: Heat Transfer
Course Code: CH-221
Course Type: Core
Contact Hours/Week: 3L+1T Course Credits: 04
Course Objectives
To impart knowledge about the fundamentals of heat transfer mechanisms in fluids
To enable the students about the applications in various design of heat transfer equipment in chemical
industries.
Unit Number Course Content Lectures
Introduction: General concept and applications of heat transfer by conduction,
UNIT-01 02L
convection and radiation.
Conduction: Fourier law, thermal conductivity in gases, liquids and solids and their
estimations, one dimensional heat conduction with and without heat generation
UNIT-02 through plane walls, cylindrical and spherical surfaces, composite layers, insulating 07L
materials: critical and optimum thickness, extended surfaces, fins and their practical
applications, unsteady state heat transfer and lumped heat model.
Convection: Heat transfer coefficients, natural and forced convection, hydrodynamic
and thermal boundary layers, laminar and turbulent heat transfer inside and outside of
tubes, dimensional analysis, individual and overall heat transfer coefficients,
UNIT-03 09L
correlations of heat transfer coefficient, condensation of mixed and pure vapors, film
wise and drop wise condensations, loading in condensers and calculation of
condensers.
Radiation: Black and gray body concept, Kirchoff’s law, Wein’s displacement law,
UNIT-04 06L
Stefan-Boltzmann law, radiation between surfaces, combined heat transfer.
Heat Exchangers: Classification and design criteria, types of heat exchangers:
double pipe, shell and tube, and plate type, fouling factors. mean temperature
UNIT-05 07L
difference, LMTD, temperature correction factor, NTU and efficiency of heat
exchangers.
Evaporation: Principle, types of evaporators: single and multiple effects, material and
UNIT-06 energy balance in evaporators, boiling point elevation, effect of liquid head, thermo 05L
compression.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Identification of heat transfer mechanisms in fluids and solids.
CO2: Describe and analyze problems involving steady state and unsteady state heat transfer.
CO3: Apply Principles of heat transfer in designing of heat exchangers and evaporators.
CO4: Assess the performance of heat exchangers and evaporators by numerical problems.
Books and References
1. Heat Transfer by J.P. Holman, 9th edition, McGraw Hill.
2. Heat Transfer: A Practical Approach by Y.A. Cengel, 4th edition, McGraw Hill, New York.
3. Unit Operations in Chemical Engineering by W.L. McCabe, and J.C. Smith, McGraw Hill, New York.
4. Process Heat Transfer by D.Q. Kern, McGraw Hill, New York.
Department of Chemical Engineering, NIT Hamirpur Page 14 of 56
Course Name: Chemical Engineering Thermodynamics-II
Course Code: CH-222
Course Type: Core
Contact Hours/Week: 3L+1T Course Credits: 04
Course Objectives
To impart knowledge about the concepts of chemical engineering thermodynamics.
To introduce the fundamental concepts relevant to different chemical process for mixtures.
To enable the students to understand the factors that cause the thermodynamic challenges in different chemical
industries.
Unit Number Course Content Lectures
Thermodynamic Properties of Fluids: Maxwell relations, relationships among the
UNIT-01 thermodynamic properties of single phase systems, residual properties, residual 06L
properties from equations of state, two phase systems.
Thermodynamics of Flow Processes: Compressible fluids, incompressible fluids,
UNIT-02 pump, compressors and ejectors, working principle and efficiency of pumps, 05L
compressors and ejectors.
Equilibrium and stability: Criteria of equilibrium, chemical potential, application of
UNIT-03 equilibrium criteria, Clausius-Clayperon equation, criteria of stability, application of 08L
stability criteria, equation related to stability.
Phase Equilibria: Critical phase equilibria, bubble point and dew point, fugacity,
composition of phases at equilibria, fugacity of pure components, fugacity charts,
effects of temperature on fugacity, Gibb’s Duhem equation in terms of activity
UNIT-04 coefficients for two component system, relating activity coefficient with composition, 10L
theoretical calculation of activity coefficient, relation for excess free energy,
thermodynamic consistency tests, Margule and Van Laar equation, various methods to
calculate Van Laar and Margule’s constants.
Chemical Reaction Equilibrium: Reaction ordinate for single & multiple reactions,
condition of equilibrium for a chemical reactions, standard states and Gibbs free energy,
UNIT-05 temperature dependence of the equilibrium constant, estimation of equilibrium rate 07L
constant, chemical equilibrium constant, homogeneous and heterogeneous gas phase
reactions.
Course Outcomes
Upon Successful completion of the course, the students will be able to
CO1: Relate and understand the various thermodynamic properties.
CO2: Describethe different criteria of equilibrium based on thermodynamics relations.
CO3: Apply principle of thermodynamics laws to determine the different equilibrium states.
CO4: Assess theimportance of chemical reaction equilibrium concepts.
Books and References
1. Chemical Engineering Thermodynamics by Y.V.C. Rao, Universities Press, Hyderabad, 1997.
2. Introduction to Chemical Engineering Thermodynamics by J.M. Smith, H.C. VanNess, and M.M. Abbott, Tata
McGraw Hill, 2010.
3. Chemical and Process Thermodynamics by B.G. Kyle, Prentice Hall PTR, New Jersey, 1999.
4. A Text book of Chemical Engineering Thermodynamics, K.V. Narayanan, PHI, 2013.
5. Thermodynamics and an Introduction to Thermostatistics by H.B. Callen, John Wiley and Sons, 1985.
Department of Chemical Engineering, NIT Hamirpur Page 15 of 56
Course Name: Industrial Pollution Abatement
Course Code: CH-223
Course Type: Core
Contact Hours/Week: 3L+ 1T Course Credits: 04
Course Objectives
To understand the important issues about industrial pollution
To impart the knowledge about the abatement principles of industrial pollution
Unit Number Course Content Lectures
Introduction: Industrial pollution, different types of wastes generated in an industry,
UNIT-01 06L
different water pollutants, air pollutants and solid wastes from industry.
Water Pollution: Identification, quantification and analysis of wastewater, classification of
different treatment methods into physico-chemical and biochemical techniques,
physicochemical methods, general concept of primary treatment, liquid-solid separation,
UNIT-02 design of a settling tank, neutralization and flocculation, disinfection, biological methods, 12L
concept of aerobic digestion, design of activated sludge process, concept of anaerobic
digestion, biogas plant layout, different unit operations and unit processes involved in
conversion of polluted water to potable standards.
Air Pollution: Classification of air pollutants, nature and characteristics of gaseous and
particulate pollutants, analysis of different air pollutants, description of stack monitoring kit
and high volume sampler, atmospheric dispersion of air pollutants, Gaussian model for
UNIT-03 prediction of concentration of pollutant down wind direction, plume and its behavior, 12L
operating principles and simple design calculations of particulate control devices, brief
concepts of control of gaseous emissions by absorption, adsorption, chemical
transformation and combustion.
Solid Wastes: Analysis and quantification of hazardous and non-hazardous wastes,
UNIT-04 6L
treatment and disposal of solid wastes, land filling, leachate treatment, incineration.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Quantify and analyze the pollution load
CO2: Analyze/design of suitable treatment for wastewater.
CO3: Model the atmospheric dispersion of air pollutants.
CO4: Selection and design of air pollution control devices.
CO5: Analyze the characteristics of solid waste and its handling & management.
Books and References
1. Environmental Engineering by H.S. Peavy, D.R. Rowe, and G. Tchobanoglous, McGraw Hill, 1985.
2. Introduction to Environmental Engineering and Science by G.M. Masters, Prentice Hall off India, 2008.
3. Wastewater Engineering by Metcalf and Eddy, Tata McGraw-Hill Education Private Limited, 2009.
4. Environmental Pollution Control Engineering by C.S. Rao, Wiley Eastern, 2010.
5. Air Pollution Control Engineering by N. De Nevers, McGraw-Hill, 2000.
Department of Chemical Engineering, NIT Hamirpur Page 16 of 56
Course Name: Chemical Technology
Course Code: CH-224
Course Type: Core
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To expose students to understand the advancement in chemical process industries and its application to
chemical engineering.
Improve their ability to read and abstract the process flow diagrams.
Equip themselves with different feed preparation, separation and purification steps involved in manufacture of
organic and inorganic chemicals.
Unit Number Course Content Lectures
Study of the following chemical industries/processes involving process details,
production trends, thermodynamic considerations, material and energy balances,
flow sheets, engineering problems pertaining to materials of construction, waste
regeneration/recycling and safety, environmental and energy.
Natural Products Processing: Gasification of coal and chemicals from coal,
Fermentation process, Sugar Industries: Manufacture of raw and refined sugar, by-
UNIT-01 products of sugar industry. Oils and Fats: Types of oil, different fatty acids, 10L
extraction of oil from seeds, oil purification, hydrogenation of oil. Manufacture of
paints and varnishes, pigments.
Soaps and Detergents: Types of soaps, soap manufacture, recovery and
purification, manufacturing of detergents.
UNIT-02 07L
Pulp and Paper industry: various pulping methods, recovery of chemicals from
black liquor, manufacture of paper, quality improvement of paper.
Chlor-alkali Industries: Manufacture of Soda ash, brine electrolysis, manufacture
UNIT-03 of caustic soda and chlorine in mercury cells, diaphragm cells, membrane cells, 03L
Bleaching powder.
Fertilizer Industries: Ammonia, nitric acid, ammonium sulphate, ammonium
chloride, urea Phosphorus, phosphoric acid, phosphatic fertilizers, calcium
UNIT-04 07L
phosphate, ammonium phosphates, nitrophosphates, sodium phosphate,
potassium chloride and potassium sulphate.
Acids: Mining of sulphur and manufacture of sulphuric acid, hydrochloric acid, nitric
UNIT-05 04L
acid.
Ceramic Industries: Types and manufacture of cement, lime, gypsum,
UNIT-06 05L
manufacture of glasses and special glasses, refractories.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Understand the processes involved in manufacturing of various inorganic and organic chemicals.
CO2: Prepare the process flow diagrams
CO3: Analyze important process parameters and engineering problems during production.
Books and References
1. Shreve’s Chemicals Process Industries by G.T. Austine, McGraw Hill.
2. Dryden’s Outlines of Chemical Technology, G.M. Rao, and M. Sittig, East West Press, New Delhi.
3. Chemical Technology by G.N. Pandey, Vol - 1, Lion Press, Kanpur.
4. Industrial Chemicals by W.L. Faith, D.B. Keyes, and R.L. Clark, Wiley.
5. Encyclopedia of Chemical Technology by Kirk, and Othmer, Wiley.
Department of Chemical Engineering, NIT Hamirpur Page 17 of 56
Course Name: Computational Lab
Course Code: CH-225
Contact Hours/Week: 2P Course Credits: 01
Course Objectives
Teach to convert chemical engineering problem into numerical code.
To give insights of computational tools such as MATLAB
To solve chemical engineering problems numerically
List of Experiments
1. Introduction to programming with MATLAB to solve chemical engineering problems.
2. Bracketing methods to find out roots such as Bisection and False-position methods.
3. Open methods to find out roots such as Newton-Raphson and Secant methods.
4. Solution of linear algebraic equations and matrices using Gauss Elimination, Gauss-Seidel etc.
5. Finding eigenvalues and eigenvectors.
6. Curve fitting and interpolation
7. Numerical integration methods such as Trapezoidal rule and Simpson’s rules
8. Numerical differentiation with MATLAB
9. Solutions of ordinary differential equations of initial value problems using Euler’s method, Runge-Kutta
methods etc.
10. Solutions of ordinary differential equations of boundary value problems using shooting methods etc.
11. Solving ODEs using finite-difference methods
12. Solving PDEs using MATLAB.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Apply numerical techniques to solve various chemical engineering problems.
CO2: Solve PDEs through MATLAB.
CO3: Analyze two-and three dimensional models through computations.
Department of Chemical Engineering, NIT Hamirpur Page 18 of 56
Course Name: Heat Transfer Lab
Course Code: CH-226
Contact Hours/Week: 2P Course Credits: 01
Course Objectives
To define the fundamental concepts to students in the area of heat transfer and its applications.
To recognize the practical significance of various parameters those are involved in different modes of heat
transfer.
To apply the knowledge of heat transfer in an effective manner for different applications.
List of Experiments
1. To determine the heat transfer coefficient of double pipe heat exchanger
2. To determine the LMTD and overall heat transfer coefficient of shell and tube heat exchanger
3. To compute the thermal resistance and thermal conductivity of a composite wall
4. To determine the thermal conductivity in forced convection apparatus
5. To study the variation of heat transfer coefficient over the surface in natural convection apparatus
6. To study the drop wise and film wise condensation
7. To determine the Stefan-Boltzman’s constant
8. To determine the emissivity of a test surface
9. To study the vertical and horizontal condenser
10. To determine the efficiency single effect evaporator
11. To determine the efficiency of plate type heat exchanger
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Identify practically different modes of heat transfer viz. conduction, convection and radiation.
CO2: Describe convective heat transfer coefficient, overall heat transfer coefficient and dimensionless numbers.
CO3: Apply principles of heat transfer to various equipment.
CO4: Study various types of heat exchangers and compare their efficiencies.
Department of Chemical Engineering, NIT Hamirpur Page 19 of 56
Course Name: Chemical Technology Lab
Course Code: CH-227
Contact Hours/Week: 2P Course Credits: 01
Course Objectives
To introduce the students some simple chemical preparations such as preparation of soaps, pigments, resins
and dye.
To teach chemical analysis and extraction techniques.
List of Experiments
1. To prepare and study the properties of soap
2. Estimation of CaO in a given cement solution
3. Preparation of Azo dye
4. Preparation of urea and phenol formaldehyde
5. Preparation of prussian blue and chrome yellow
6. Preparation of pigments (barium white, malchite green and chromium oxide green)
7. To prepare phenol formaldehyde resin (Bakelite)
8. Extraction of oil from any seed material using Soxhlet apparatus
9. Estimation of moisture content of a given sample by Dean and Stark apparatus.
10. Estimation of carbon residue of a given sample using Conradson apparatus.
11. Estimation of cloud and pour point of a given sample
12. Estimation of flash point, fire point, smoke point of oils
13. Determination of aniline point of a given oil sample.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Conduct experimental procedure for manufacture of soap, organic chemicals, dye, etc.
CO2: Handle different apparatus and learn the skill in using instruments for analysis.
Department of Chemical Engineering, NIT Hamirpur Page 20 of 56
Course Name: Mass Transfer-I
Course Code: CH-311
Course Type: Core
Contact Hours/Week: 3L+ 1T Course Credits: 04
Course Objectives
To impart knowledge about the basics of mass transfer processes.
To introduce the fundamental laws and theories for basic mass transfer processes.
To enable the student to learn about the gas-liquid equilibrium operations.
Unit Number Course Content Lectures
Diffusion: Classification of mass transfer operation, choice of separation
methods,steady state molecular diffusion in fluids at rest and in laminar flow,
UNIT-01 08L
molecular diffusion in gases, molecular diffusion in liquids, diffusivity in liquids and
gases, momentum and heat transfer in laminar flow.
Mass Transfer Coefficient & Theories: Local and overall mass transfer coefficient,
heat and mass transfer analogy, eddy diffusivities, dimensionless numbers and their
UNIT-02 07L
significance, film theory, penetration theory, surface renewal theories, combination
film theory and surface stretch theory.
Interphase Mass Transfer: Equilibrium, local two phase mass transfer coefficients,
local overall mass transfer coefficients, material balance for co current and counter
UNIT-03 06L
current processes, and concept of ideal stage and stage efficiencies, continuous
contact equipment.
Gas Absorption and Stripping: Choice of solvent, estimation of number of ideal
stages – graphical and analytical methods, minimum solvent flow rate, significance of
UNIT-04 absorption factor, number of transfer units and height of a transfer unit (NTU & HTU) 07L
concepts, packed column for absorption, HETP, rate of absorption, height of column
based on condition in gas film and liquid film, height based on overall coefficients.
Humidification: Wet and dry bulb hygrometry, psychometric chart and its use,
UNIT-05 04L
cooling towers: classification, construction, operation and calculation,
Drying: Equilibrium in drying, batch drying and rate of batch drying, time of drying,
UNIT-06 04L
drying rate calculation, drying equipment.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Familiar with the basic phenomenon, principles and theories of mass transfer
CO2: Determine diffusivity and mass transfer coefficient in gases and liquids
CO3: Describe the phenomena involving interphase mass transfer in industrial processes
CO4: Apply mathematical and design concepts of mass transfer in gas-liquid systems like absorption, humidification,
drying processes
Books and References
1. Mass Transfer Operations by R.E. Treybal, McGraw Hill, 1980.
2. Principles of Mass Transfer and Separation Processes by B.K. Dutta, PHI, 2006.
3. Transport Processes and Separation Process Principles by C.J. Geankopolis, Prentice Hall of India, Eastern
Economy Edition, 2004.
4. Chemical Engineering by J.M. Coulson, and J.F. Richardson, Vol. 2 & 5, McGraw Hill, 1999.
Department of Chemical Engineering, NIT Hamirpur Page 21 of 56
Course Name: Chemical Reaction Engineering-I
Course Code: CH-312
Course Type: Core
Contact Hours/Week: 3L+ 1T Course Credits: 04
Course Objectives
To make the student understand principles and practices followed in chemical industries with respect to reactor
design and operation.
To enable the students to analyze the kinetic data, and to estimate the kinetic parameters.
Unit Number Course Content Lectures
Introduction: Kinetics of homogeneous reactions, concentration and temperature
dependent term of rate equation, interpretation of batch reactor: constant volume
UNIT-01 batch reactor, integral and differential method of analysis of data, method of half life 07L
and initial rates, series and parallel reactions, reversible reactions, variable volume
batch reactor, temperature and reactions rate.
Reactor Design: Ideal batch reactor, CSTR, plug flow reactor, holding and space
time, design for single reactions, size comparison (analytical and graphical method,
UNIT-02 08L
plug flow reactors in series & parallel, mixed reactor in series, recycle and
autocatalytic reactions
Design for Multiple Reactions: Reactions in parallel and series in CSTR and plug
UNIT-03 05L
flow reactor, conversion, yield and selectivity.
Temperature and Pressure Effect: General design procedure, optimum
UNIT-04 temperature progression, adiabatic operation, non-adiabatic operation, semi batch 05L
reactors
Residence Time Distribution (RTD): Fundamentals of non-ideal reactors;
UNIT-05 measurement and characterization of RTD: C curve, E curve, F curve, mean 05L
residence time, different moments; RTD for ideal reactor (batch, CSTR, PFR);
Non-ideal Reactor Modeling Using RTD: Zero parameter model: segregation
UNIT-06 06L
model and maximum mixedness model, tanks in series model, dispersion model.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Derive the rate law for elementary and non-elementary reactions
CO2: Determine the kinetics of chemical reaction from the data using integral, differential method of analysis
CO3: Design of reactors for conducting the homogeneous reactions under isothermal conditions
CO4: Compare the performance of ideal reactors
CO5: Determine the mean residence time and standard deviation using the RTD data
CO6: Analyze the performance of non-ideal reactors using various models.
Books and References
1. Elements of Chemical Reaction Engineering by H.S. Fogler, PHI, 2010.
2. Chemical Reaction Engineering by O. Levenspiel, Wiley, India, 2007.
3. Chemical Reactor Analysis and Design by G.F. Froment, K.B. Bischoff, and J.D. Wilde, Wiley, India, 2011.
4. Chemical Engineering Kinetics by J.M. Smith, McGraw-Hill, 1970.
5. Introduction to Chemical Engineering Kinetics and Reactor Design, C.G. Hill, and T.W. Root, Wiley, India, 2014.
Department of Chemical Engineering, NIT Hamirpur Page 22 of 56
Course Name: Process Equipment Design-I
Course Code: CH-313
Course Type: Core
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To understand various process codes & standards to perform process design/equipment design calculations.
To address the stress and strain produced in different parts of the equipment such as shell, head, support, etc.
due to operating conditions of the process.
To perform process equipment design calculations manually.
Unit Number Course Content Lectures
Equipment Design Preliminaries: Principles involved in the design and
construction of equipment, materials of construction, design codes, pressure,
UNIT-01 temperature, factor of safety, corrosion allowance, weld joint efficiency factor, design 06L
loadings, Poisson’s ratio, dilation of pressure vessels, stress concentration, thermal
stresses, criteria of failure.
Design of Pressure Vessels/ Storage Tanks: Introduction to Indian Standards for
storage tanks and their use to design cylindrical and spherical vessels under internal
UNIT-02 07L
pressure, fixed roof and open roof tanks, design of different heads such as flat cover
head, conical head, torispherical head and ellipsoidal head.
Design of Non-standard Flange, Pipe Fitting and Joints: Types of flange and
selection, specification of standard flanges, design of non-standard flanges including
UNIT-03 06L
gasket, design of bolts, screws, welded and riveted joints, design of different pipe
fittings
Design of Supports: Design of skirt, lug and saddle supports for vertical and
UNIT-04 06L
horizontal vessels.
Design of Thick-walled High-pressure Vessels: Stresses in a thick cylinder,
UNIT-05 06L
theories of elastic failure.
Equipment Fabrication and Testing: Design of welded joints, post weld treatment,
UNIT-06 05L
inspection and non-destructive testing of equipment.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Apply the Indian Standards to perform process design/equipment design calculations.
CO2: Calculate stress and strain induced in different parts of the equipment such as shell, head, support, etc. due to
operating conditions of the process.
CO3: Apply step-by-step mechanical design aspects to design any process equipment.
Books and References
1. Chemical Equipment Design by B.C. Bhattacharya, CBS Publisher, 1985.
2. Process Equipment Design by L.E. Brownell, and E.H. Young, John Wiley & Sons, 2009.
3. Joshi’s Process Equipment Design by V.V. Mahajani, and S.B. Umarji, 5th edition, Laxmi Publications, 2016.
4. Chemical Engineering by R.K. Sinnot, J.M. Coulson, and J.F. Richardson, Vol.-6, Butterworth Heinemann, 1998.
5. Applied Process Design for Chemical and Petrochemical Plants by E.E. Ludwig, Vol. -1, 2 & 3, Gulf Publishing
Company, 1995.
Department of Chemical Engineering, NIT Hamirpur Page 23 of 56
Course Name: Process Dynamics and Control
Course Code: CH-314
Course Type: Core
Contact Hours/Week: 3L + 1T Course Credits: 04
Course Objectives
To analyze the system behavior for the design of various control schemes
To impart the knowledge of different process instruments.
Unit Number Course Content Lectures
Introduction: General principles of process control, time domain, Laplace domain
UNIT-01 and frequency domain dynamics and control. 04L
Linear Open-loop Systems: Laplace domain analysis of first and second orders
systems, linearization, response to step, pulse, impulse and ramp inputs, first and
UNIT-02 second order systems, thermocouple, level tank, mixing tank, U-tube manometer. 08L
Interacting and non-interacting systems, distributed and lumped parameter systems,
dead time.
Linear Closed-loop Systems: Controllers and final control elements, different
types of control valves and their characteristics, development of block diagram,
UNIT-03 08L
transient response of simple control systems, stability in Laplace domain, root locus
diagram.
Frequency Response: Frequency domain analysis, control system design by
UNIT-04 frequency response, Bode stability criterion, Nyquist stability criteria, design of 08L
controllers, different methods of tuning of controllers.
Process Applications: Advanced control techniques; feed forward, feedback,
UNIT-05 cascade, ratio, Smith predictor and inverse response compensator, application to 08L
equipment; distillation columns, reactors.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Set up a model, analyse and solve the first and second order system for its dynamic behaviour.
CO2: Evaluate the process stability in Laplace domain.
CO3: Design control system using frequency response analysis
CO4: Identify advanced control techniques for chemical process.
Books and References
1. Process Systems Analysis and Control by D.R. Coughanowr, and S.E. LeBlanc, McGraw Hill, 2009.
2. Chemical Process Control: An Introduction to Theory and Practice by G. Stephanopoulous, Prentice Hall of
India, 1984.
3. Automatic Process Control by D.P. Eckman, Wiley Eastern Ltd., New Delhi, 2009.
4. Process Control by P. Harriott, Tata McGraw Hill, 1972.
Department of Chemical Engineering, NIT Hamirpur Page 24 of 56
Course Name: Chemical Reaction Engineering Lab
Course Code: CH-315
Contact Hours/Week: 2P Course Credits: 01
Course Objectives
To learn about the different types of reactors
To learn about carrying out reaction in different types of reactors to verify theoretical principles
To learn about the design of reactors
To learn about the estimation of reaction kinetic parameters practically
List of Experiments
1. Study of a non-catalytic homogeneous reaction in a CSTR under isothermal conditions.
2. Study of a non-catalytic homogeneous reaction in a PFR under isothermal conditions.
3. Study of a non-catalytic homogeneous reaction in a PBR under isothermal conditions.
4. Study of Residence time distribution (RTD) in a PFR.
5. Study of Residence time distribution (RTD) in a PBR.
6. Study of Residence time distribution (RTD) in CSTR.
7. Study of a non-catalytic homogeneous reaction in a Batch Reactor under isothermal conditions.
8. Study of hydrodynamics of trickle bed reactor
9. Study of spinning basket reactor
10. Study of a adiabatic batch reactor
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Learn how to experimentally verify various theoretical principles
CO2: Visualize practical implementation of chemical engineering equipment
CO3: Develop experimental skill
Department of Chemical Engineering, NIT Hamirpur Page 25 of 56
Course Name: Process Dynamics and Control Lab
Course Code: CH-316
Contact Hours/Week: 2P Course Credits: 01
Course Objectives
To learn about basics of control
To learn about pneumatic valve characteristics
To study level, temperature and flow control
List of Experiments
1. To study the response of thermometer
2. To study the dead weight pressure gauge
3. To study the control valve characteristics
4. To study the interacting and non-interacting systems
5. To study the temperature controller
6. To study the flow controller
7. To study the study of PI and IP converter
8. To study the level control trainer
9. To study the cascade control trainer
10. To study the pressure controller
11. To study the ratio controller
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Find parameters of two tank system and verify PI-IP conversion
CO2: Control level, flow and temperature using control system
CO3: Draw valve characteristic curve
Department of Chemical Engineering, NIT Hamirpur Page 26 of 56
Course Name: Industrial Pollution Abatement Lab
Course Code: CH-317
Contact Hours/Week: 2P Course Credits: 01
Course Objectives
To characterize the wastewater sample.
To monitor the air quality.
List of Experiments
1. Determination of total solid, total dissolved solid and Total Suspended Solid for a Given Sample.
2. Determination of total acidity and total alkalinity.
3. Determination of total hardness and estimation of chlorides.
4. Determination of Chemical Oxygen Demand (COD) of a given sample.
5. Determination of Dissolved oxygen (DO) in various samples by Winkler method.
6. Determination of Biological Oxygen Demand (BOD) from a given waste water sample.
7. Determination of Sludge Volume Index (SVI) of a given waste water sample.
8. Determination of phosphorous in waste water sample.
9. Estimation of fluoride in a given sample.
10. Determination of nitrite and nitrate nitrogen in waste water sample.
11. Determination of ammonical and organic nitrogen in waste water sample.
12. High volume sampler to measure the air quality.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Determine the physical parameters of wastewater sample.
CO2: Determine the chemical and biological parameters of wastewater sample.
CO3: Measure the air quality.
Department of Chemical Engineering, NIT Hamirpur Page 27 of 56
Course Name: Mass Transfer-II
Course Code: CH-321
Course Type: Core
Contact Hours/Week: 3L+1T Course Credits: 04
Course Objectives
To impart knowledge about the various mass transfer equilibrium.
To introduce the industrially important mass transfer processes such as distillation, extraction, leaching etc.
To enable the student to solve design problems related to mass transfer equipment.
Unit Number Course Content Lectures
Distillation: Mass Transfer equilibria, Raoult’s Law and Dalton’s law. partial
vaporization and partial condensation, relative volatility,differential distillation and flash
UNIT-01 distillation, steam distillation, McCabe–Thiele and Ponchon Savarit methods, Fenske, 11L
Underwood and Gilliland equations, total reflux, minimum and optimum reflux ratios,
multiple feeds and side streams.
Liquid–Liquid Extraction: Ternary phase diagrams and solvent selection, single
UNIT-02 stage& multistage cross current, co-current and counter current extraction operation 07L
for immiscible and miscible solvents, batch and continuous contact extractors.
Leaching: Solid-liquid equilibria,single stage & multistage cross current, co-current
UNIT-03 and countercurrent leaching operations, supercritical fluid extraction, equipment for 06L
leaching.
Adsorption: Introduction and the nature of adsorbent, adsorption equilibria,
UNIT-04 Langmuir, Freundlich, BET and Gibbs isotherms, potential theory, adsorption 06L
equipment, pressure and temperature swing adsorption,ion-exchange equilibria.
Crystallization: Formation of nuclei, nuclei growth and properties of crystals, effect of
UNIT-05 impurities on crystals formation, effect of temperature on solubility, caking of crystals, 06L
yield of crystals, crystallizers.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Familiar with the various types of mass transfer equilibrium.
CO2: Determine the No. of plates, height of column using analytical and graphical techniques in distillation and,
extraction and leaching operations.
CO3: Select solvent for extraction and leaching operations.
CO4: Solve related to adsorption and crystallization.
Books and References
1. Mass Transfer Operations by R.E. Treybal, McGraw Hill, 1980.
2. Principles of Mass Transfer and Separation Processes by B.K. Dutta, Prentice Hall of India, 2006.
3. Transport Processes and Separation Process Principles by C.J. Geankopolis, Prentice Hall of India, Eastern
Economy Edition, 2004.
4. Chemical Engineering by J.M. Coulson, and J.F. Richardson, Vol. – 2 & 5, McGraw Hill, 1999.
5. Unit Operations of Chemical Engineering by W. McCabe, J. Smith, and P. Harriott, McGraw Hill, 2017.
Department of Chemical Engineering, NIT Hamirpur Page 28 of 56
Course Name: Chemical Reaction Engineering-II
Course Code: CH-322
Course Type: Core
Contact Hours/Week: 3L+ 1T Course Credits: 04
Course Objectives
To provide the students with principles and kinetic tools useful in analyzing the rates of chemical reactions for
heterogeneous systems.
To enable the students to do the design of heterogeneous reactor systems.
Unit Number Course Content Lectures
Catalysis: Theories of heterogeneous catalysis, classification of catalysts, catalyst
preparation, promoter and inhibitors, catalysts deactivation. steps in a catalytic
UNIT-01 08L
reaction, synthesizing a rate law, mechanism and rate limiting step, heterogeneous
data analysis for reactor design, reactor design.
Solid Catalysts: Determination of surface area, void volume and solid density, pore
UNIT-02 05L
volume distribution.
Internal Diffusion: quantitative aspects of pore diffusion controlled reactions (single
UNIT-03 cylindrical pore), effective diffusivity, mole balance for the elementary slice of catalyst 08L
pore, Thiele Modulus and internal effectiveness factor, overall effectiveness factor.
External Diffusion: Concept of external diffusion control, external resistance to
UNIT-04 mass transfer, mass transfer to a single particle, mass transfer limited reaction in a 05L
packed bed, shrinking core model (catalyst regeneration).
Introduction to Fluid Reactions: Kinetic regimes for mass transfer and reaction,
UNIT-05 film conversion parameter, clues to the kinetic regime from solubility data, clues to 05L
the kinetic regime from equipment, applications to design.
Fluid-Particle Reactions: Selection of model, unreacted core model for spherical
UNIT-06 particles, diffusion through gas film control and diffusion through ash layer control, 05L
chemical reaction control, design.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Derive the rate law for a catalytic reaction using the kinetic data
CO2: Determination of surface area and pore size of catalyst.
CO3: Determine the internal and overall effectiveness factors.
CO4: Understand the effect of velocity, particle size and fluid properties on rate of reactions controlled by mass
transfer.
CO5: Design fixed bed reactors involving chemical reactions with mass transfer.
CO6: Analyze the fluid particle reactions using the models.
Books and References
1. Elements of Chemical Reaction Engineering by H.S. Fogler, PHI, 2010.
2. Chemical Engineering Kinetics by J.M. Smith, McGraw-Hill, 1970.
3. Chemical Reactor Analysis and Design by G.F. Froment, K.B. Bischoff, and J.D. Wilde, Wiley, India, 2011.
4. Chemical Reaction Engineering by O. Levenspiel, Wiley, India, 2007.
5. Introduction to Chemical Engineering Kinetics and Reactor Design by C.G. Hill, and T.W. Root, Wiley, India,
2014.
Department of Chemical Engineering, NIT Hamirpur Page 29 of 56
Course Name: Process Equipment Design-II
Course Code: CH-323
Course Type: Core
Contact Hours/Week: 3L +1T Course Credits: 04
Course Objectives
To apply the basic principles/concepts learned in the subjects of Fluid Mechanics, Heat Transfer, Mass Transfer,
and Mechanical Operation in the mechanical design of chemical process equipment.
To develop the skill to select and design the appropriate process equipment for the required unit or process
operation.
To analyze and evaluate the performance of existing equipment.
Unit Number Course Content Lectures
Process Design of Heat Exchanger: Heat exchanger classification, thermal design
UNIT-01 consideration, design procedure of shell and tube heat exchanger for two phase heat 07L
transfer, design of condenser and reboiler,
Mechanical Design of Heat Exchanger: Design standards of shell and tube heat
exchanger, design temperature and pressure, materials of construction, design of
UNIT-02 05L
different components (shell, channel cover, tube, baffles, nozzles etc.) of shell and
tube heat exchanger.
Design of Evaporator: Thermal design of single and multiple effects evaporators,
calculation of tube-side and shell-side pressure drop; calculation of intermediate
UNIT-03 06L
temperatures of multiple effects evaporator; estimation of overall heat transfer
coefficients, mechanical design of standard vertical short tube evaporator.
Design of Dryer: Calculation of process design variables of rotary dryer such as
inlet and exit moisture contents of the solid; the critical & equilibrium moisture
UNIT-04 06L
contents; temperature and humidity of the drying gas, mechanical design of rotary
dryer.
Process Design of Mass Transfer Columns: Effect of vapor flow conditions of tray
UNIT-05 06L
design, column sizing approximation, detailed design of tray.
Mechanical Design of Mass Transfer Columns: Column construction and
UNIT-06 internals, different stresses (axial, circumferential and compressive) induced in 04L
column and its calculations.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Integrate the knowledge acquired from chemical engineering courses in the design of equipment.
CO2: Design heat exchangers, condensers, reboilers, evaporators and dryers.
CO3: Design and analyze tray columns.
CO4: Apply mechanical design aspects to process equipment.
Books and References
1. Chemical Equipment Design by B.C. Bhattacharya, CBS Publisher, 1985.
2. Process Heat Transfer by D.Q. Kern, McGraw Hill, 2001.
3. Joshi’s Process Equipment Design by V.V. Mahajani, and S.B. Umarji, 5th edition, Laxmi Publications, 2016.
4. Chemical Engineering by R.K. Sinnot, J.M. Coulson, and J.F. Richardson, Vol.- 6, Butterworth Heinemann,
1998.
5. Applied Process Design for Chemical and Petrochemical Plants by E.E. Ludwig, Vol. - 1, 2 & 3, Gulf Publishing
Company, 1995.
6. Perry’s Handbook of Chemical Engineering by D.W. Green, and R.H. Perry, McGraw Hill, 1997.
Department of Chemical Engineering, NIT Hamirpur Page 30 of 56
Course Name: Process Modeling and Simulation
Course Code: CH-324
Course Type: Core
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To provide necessary training for process synthesis using simulation tools.
To understand knowledge of fundamental principles and basic laws of modeling and approach for modeling.
To develop mathematical models of chemical engineering systems using fundamental conservation laws.
Unit Number Course Content Lectures
Introduction: Modeling and simulation, classification, uses of mathematical models and tools,
physical and mathematical modeling, deterministic and stochastic process, types of modeling
UNIT-01 05L
equations, and classification of models (lumped parameter models, distributed parameter
models).
Mathematical Models: principles of model formulation, fundamental laws- continuity equation,
UNIT-02 energy equation, equations of motion, transport equations, equations of state, equilibrium and 06L
kinetics, constitutive relationships, dimensionless analysis, degree-of-freedom analysis.
Lumped Parameter Models: Series of isothermal constant holdup CSTRs, CSTRs with
variable holdups, non-isothermal CSTR, batch reactor, batch distillation with holdup, ideal
UNIT-03 11L
binary distillation column, gas absorber,interacting and non-interacting tanks, model for heat
exchanger, gravity flow tank, single and multiple effect evaporator systems.
Distributed Parameter Models: Convective problems, laminar flow of Newtonian liquid in a
UNIT-04 06L
pipe, diffusive problems, combined convective and diffusive problems
Simulation: Implementation of the models and numerical methods using MATLAB/Simulink,
introduction and use of process simulation software (ASPEN) for flow sheet simulation.
UNIT-05 08L
Simulation of models such as isothermal CSTR, non-isothermal CSTR, batch reactor, heat
exchangers.
Course Outcomes
Upon successful completion of the course, students will be able to
CO1: Develop an understanding how the simulators work and mathematical model of chemical engineering systems from
fundamental laws.
CO2: Write computer program to simulate the model.
Books and References
1. Process Modeling Simulation and Control for Chemical Engineers by W.L. Luyben, McGraw Hill, 2013.
2. Process Plant Simulation by B.V. Babu, Oxford University Press, 2004.
3. Introduction to Chemical Engineering Analysis by T.W.F. Russell, and M.M. Denn, Wiley, 1972.
4. Process Dynamics - Modelling, Analysis and Simulation by B.W. Bequette, PHI International, 2003.
5. Chemical Reactor Design for Process Plants by H.F. Rase, Vol.-2: Case Studies and Design Data, John Wiley,
New York, 1997.
Department of Chemical Engineering, NIT Hamirpur Page 31 of 56
Course Name: Mass Transfer Lab.
Course Code: CH-325
Contact Hours/Week: 2P Course Credits: 01
Course Objectives
To make students understand and apply the basics of mass transfer.
To provide hands-on experience to the students in working with Stefan tube, VLE set-up, Cooling tower, Plate and
packed column, welted wall column etc.
To enable the student to report and analyze data obtained from different set-up.
List of Experiments
1. Determination the number of theoretical plates in sieve plate distillation column.
2. Distillation in a packed bed column.
3. Study of heat and mass transfer in water cooling tower.
4. Study the dissolution of benzoic acid with and without chemical reaction.
5. Measurement of diffusivity for organic solvents using Stefan tube.
6. Estimation of mass transfer coefficient in wetted wall column.
7. Estimation of distillation characteristics of petroleum oils/organic solvents.
8. Determination of extraction coefficient for liquid-liquid extraction in a packed tower.
9. Determination of leaching coefficient solid -liquid extraction study in packed column.
10. To study the drying characteristics curve under constant drying condition in rotary vacuum or tray dryer.
11. To study and verify the Raleigh equation for batch distillation.
12. To determine the mass transfer coefficient for absorption in packed column
13. To study the humidification and dehumidification characteristics.
14. Estimation of flux and separation factor in membrane filtration (UF/NF)
15. To estimate the crystallization efficiency in batch crystallizer.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Determine diffusivity and mass transfer coefficient for various systems.
CO2: Describe phase, VLE diagrams, batch distillation, humidification operation and drying rate curve.
CO3: Apply principles of McCabe-Thiele to estimate the No. of plates in plate column and HETP for packed column.
CO4: Estimate the cooling tower characteristics, efficiency of liquid-liquid and solid-liquid extractors.
Department of Chemical Engineering, NIT Hamirpur Page 32 of 56
Course Name: Process Simulation Lab
Course Code: CH-326
Contact Hours/Week: 2P Course Credits: 01
Course Objectives
To introduce students to solve process simulation problems using MATLAB and ASPEN-Plus for Chemical
Engineering problems
List of Experiments
1. Introduction and Stepwise Aspen Plus simulation: flash drum examples
2. Steady state and dynamic simulation of CSTR using ASPEN HYSYS.
3. Steady state and dynamic simulation of PFR using ASPEN HYSYS.
4. Steady state and dynamic simulation of multicomponent distillation column using ASPEN HYSYS
5. Simulation of chemical plant using ASPEN Plus
6. Dynamics and control using ASPEN dynamics
7. Solving ODE, linear and non-linear algebraic equations using MATLAB
8. SIMULNIK for solving chemical engineering and control problems
9. Modeling and simulation of shell and tube/plate type heat exchanger using programming language
10. Diffusion of species in the micro-fluidic devices
11. Basic CFD problems in ANSYS and/or COMSOL software
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Run the computational software to solve physical problems
CO2: Learn industrial processes using software.
CO3: Correlations between experimental, numerical and theoretical results
Department of Chemical Engineering, NIT Hamirpur Page 33 of 56
Course Name: Process Plant Design and Economics
Course Code: CH-411
Course Type: Core
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
● To teach basic concepts of a chemical process and plant design.
● To understand concepts of economic balances and economic analysis of process plant.
● To familiarize with the commercial flowsheeting software (ASPEN Plus®) to simulate chemical processes.
Unit Number Course Content Lectures
Process Selection, Strategy for Synthesis and Analysis: Aspects of process design, pre-
project objectives, project classification, block flow diagram (BFD), process flow diagram (PFD),
UNIT-01 piping and instrumentation diagram (P&ID), conceptual design and synthesis of a process flow 04L
diagram, development of PFD from generic BFD for hydrodealkylation of toluene (HDA) case
study using ASPEN HYSYS/ ASPEN Plus®.
General Plant Design Considerations: Location, layout, site selection, general service facilities,
UNIT-02 different development stages starting from site selection till plant commissioning, environmental 05L
consideration, plant design of HDA case study.
Process Economics: Estimation of capital costs, purchased equipment costs, the total capital
cost of a plant, bare module cost-base and non-base conditions, estimation of manufacturing
UNIT-03 08L
costs, cost of labor, utility cost, raw material costs, depreciation, annuity, time value of money,
different process/ project profitability measures, profitability measures of HDA case study.
Hierarchical Approach in Process Design: Batch versus continuous processes, comparative
analysis, input information, decisions for input-output structure, overall material balance, stream
costs, process alternatives, decision for the recycle structure, equilibrium limitations,
modifications of reactor design for recycle, equipment costs associated with recycle, overall
UNIT-04 10L
economic potential of process with recycle, general structure of the separation system, location of
vapor and liquid recovery system in the process, sequencing of non-integrated distillation
columns for minimum vapor load, thermal coupling of columns, application of hierarchical
approach in different case studies.
Heat Exchanger Networking: First law analysis, cascade diagrams, temperature-enthalpy
UNIT-05 diagrams, grand composite curve, multiple utilities, area estimates, design of MER Networks, 06L
loops and paths, stream splitting, heat and power integration.
Cost Diagrams and Quick Screening of Process Alternatives: Concept of cost diagram, quick
UNIT-06 assessment of cost distribution, cost allocation procedures, lumped cost diagram, screening of 03L
process alternatives with cost diagrams using design heuristics.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Develop a chemical process from scratch.
CO2: Perform preliminary feasibility study of the proposed chemical plant.
CO3: Perform a complete economic analysis of the proposed chemical plant to calculate total capital investment, product cost
and profitability of the overall process.
CO4: Use commercial flowsheeting software to simulate chemical processes.
Books and References
1. Plant Design and Economics for Chemical Engineers by M.S. Peters, K.D. Timmerhaus, and R.E. West, 5th edition,
McGraw Hill, 2017.
2. Conceptual Design of Chemical Processes by J.M. Douglas, McGraw Hill, 1988.
3. Product & Process Design Principles: Synthesis, Analysis, and Evaluation by W.D. Seider, J.D. Seader, and D. R. Lewin,
2nd edition, Wiley-India Edition, 2004.
4. Chemical Engineering by R.K. Sinnot, J.M. Coulson, and J.F. Richardson, Vol.-6, Revised 2nd edition, Butterworth-
Heinemann, 1996.
5. Analysis, Synthesis, and Design of Chemical Processes by R. Turton, R.C. Bailie, W.B. Whiting, J.A. Shaeiwitz, and D.
Bhattacharyya, 4th edition, Prentice Hall, 2013.
6. Ludwig’s Applied Process Design for Chemical and Petrochemical Plants by A.K. Coker, Vol.-1, 2 & 3), Gulf Professional
Publishing (Vol. -1: 2007; Vol. -2: 2010; Vol- 3: 2001).
Department of Chemical Engineering, NIT Hamirpur Page 34 of 56
Course Name: Petroleum Refining and Petrochemical Engineering
Course Code: CH-412
Course Type: Core
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To impart knowledge about petroleum refineries and various operations carried out in it.
To introduce the fundamental of various aspects of petrochemical engineering and petroleum products.
To enable the student to work in a petroleum refinery as chemical engineering professional.
Unit Number Course Content Lectures
Introduction to Petroleum Industry: Scope and purpose of refining; global and
Indian refining scenario, practice and prospect. An overview of the entire spectrum of
UNIT-01 06L
the refinery products, physiochemical characteristics of petroleum and petroleum
products, refinery configuration development.
Refinery Distillation Processes: Classification of crude oil,desalting and stabilization
of crude, fractional distillation of crude oil, ASTM, TBP and EFV
UNIT-02 08L
distillation,atmospheric distillation unit, vacuum distillation unit, degree of separation
(5-95 gap) and degree of difficulty of separation (∆t 50), Packie charts.
Fuel Refining: Cracking, coking, reforming, alkylation, isomerisation, polymerization,
UNIT-03 sweetening, visbreaking, hydroprocessing: hydro cracking, hydro treating, hydro 09L
finishing.
Petrochemicals:Refinery feedstock;nature and effect of different types of refinery
feedstock and impurities on refinery configuration and operation, natural gas,
UNIT-04 production of petrochemical precursors - synthesis gas, hydrogen, acetylene, 05L
ethylene, propylene, butylene, aromatics and naphthenes, petrochemical derivatives
and products.
Polymer Based Industries and Their Characteristics: Plastic; production of
thermoplastic and thermosetting resins such as polyethylene, polypropylene, phenolic
UNIT-05 resins and epoxy resins. Polymers and their applications in engineering practice. 05L
Polyamides, polyesters and acrylics from monomers. Production of natural and
synthetic rubbers.
UNIT-06 Coal: Gasification of coal and chemicals from coal. 03L
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Identify the key problems associated with smooth operation of petroleum refinery
CO2: Describe various types of solution to problems normally encountered in refineries
CO3: Apply principles of distillation, product up gradation, catalysis and polymer science in industries
CO4: Assess the overall performance of a petroleum refinery
Books and References
1. Petroleum Refinery Engineering by W. L. Nelson, McGraw-Hill, 1961.
2. Petroleum Refinery Distillation by R.N. Watkins, Gulf Publishing, 1979.
3. Modern Petroleum Refining Processes by B.K.B. Rao, Oxford and IBH Publishing, New Delhi, 1990.
4. Fundamentals of Petroleum and Petrochemical Engineering byU. Ray Chaudhuri, CRC Press, 2010.
Department of Chemical Engineering, NIT Hamirpur Page 35 of 56
Course Name: Engineering Economics and Accountancy
Course Code: HS-404
Course Type: Core
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To impart knowledge about the Economics and its applicability to the Engineers
To introduce the fundamental concepts of economics
To enable the students to understand the factors that causes the changes in economic conditions of the entrepreneur
Unit Number Course Content Lectures
UNIT-01 Introduction to Engineering Economics: Definitions, Nature, Scope and application; 06L
Difference between Micro Economics and Macro Economics; Theory of Demand &
Supply: Meaning, Determinants, Law of Demand, Elasticity of demand, Demand
Forecasting, Law of Supply, Equilibrium between Demand & Supply.
UNIT-02 Production and Cost: Production functions, lsoquant, Least Cost combination, Laws of 06L
Returns to Scale. Economics and Diseconomies of Scale of production, Cost and Cost
curves, Revenue and Revenue curve, Break even analysis.
UNIT-03 Costing and Appraisal: Cost elements, Economic cost, Accounting cost, Standard 05L
cost, Actual cost, Overhead cost, Cost control, Criteria of project appraisal, Social cost
benefit analysis
UNIT-04 Markets: Meaning, Types of Markets, Characteristics (Perfect Competition, Monopoly, 05L
Monopolistic Competition, Oligopoly) Price and Output Determination; Product
Differentiation; Selling Costs; Excess Capacity.
UNIT-05 Money: Meaning, Functions, Types; Monetary Policy- Meaning, Objectives, Tools; 04L
Fiscal Policy:-Meaning, Objectives, Tools.
Banking: Meaning, Types, Functions, Central Bank: its Functions, concepts CRR,
Bank Rate, Repo Rate, Reverse Repo Rate, SLR.
UNIT-06 Depeciation: Meaning of depreciation, causes, object of providing depreciation, factors 04L
affecting depreciation, Methods of Depreciation: Straight line method, Diminishing
balance method, Annuity method and Sinking Fund method
UNIT-07 Financial Accounting: Double entry system (concept only), Rules of Double entry 06L
system, Journal(Sub-division of Journal) , Ledger, Trial Balance Preparation of final
accounts-Trading Account. Profit and Loss account, Balance Sheet.
Course Outcomes
Upon successful completion of the course, the students will be able to
CO1: Identify the challenges of the economy as entrepreneur/manufacturer as well as consumer
CO2: Describe the economic system at the micro and macro level
CO3: Apply principles of economics and accountancy in the professional, personal and societal life
CO4: Assess the role of engineering economics and accounting in attaining economic efficiency
Books and References
1. Principles of Micro Economics by Mceachern & Kaur, Cengage Publication.
2. Managerial Economics by Craig Peterson & W Cris Lewis, PHI Publication.
3. Modern Microeconomics by A. Koutsoyiannis, Macmillan.
4. Managerial Economics Theory and Applications by D. M.Mithani. Himalaya Publication House.
5. Fundamental of Managerial Economics Mark Hirschey, South Western Educational Publishing.
6. Engineering Economics by Degramo, Prentice Hall.
7. Financial Accounting–A Managerial Perspective by R. Narayanaswamy, PHI.
8. Introduction to Accounting by J.R. Edwards & Marriot, Sage Publication.
9. Cost Accounting by Jawahar Lal, Tata McGraw Hill.
10. Project Planning Analysis, Selection, Implementation and Review by Prasanna Chandra, Tata McGraw Hill
Department of Chemical Engineering, NIT Hamirpur Page 36 of 56
Course Name: Industrial Safety and Hazard Management
Course Code: CH-421
Course Type: Core
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To highlight the importance of industrial safety and measures in order to prevent accidental damage
To explain significant disaster observed in different parts of the world with understanding of the properties of toxic
materials
To deal with fire and explosion and concepts to prevent them
To obtain the checklist for process hazards and their safety review
Unit Number Course Content Lectures
Introduction: Safety program, engineering ethics, concept of loss prevention,
UNIT-01 accident and loss statistics, acceptable risks, nature of accident process, inherent 06L
safety, significant disaster in India, England, Texas, Italy, Florida and Georgia
Toxicology: Toxic materials and their properties, toxicants entry route, dose versus
UNIT-02 response, models for dose and response curves, threshold limit values, national fire 06L
protection association diamond
Industrial Hygiene: Industrial hygiene anticipation and identification, industrial
UNIT-03 06L
hygiene evaluation, hygiene control
Fires and Explosion: Fire triangle, distinction between fires and explosion,
definitions, flammability characteristics of liquid and vapors, LOC and inerting,
flammability diagram, ignition energy, auto ignition, auto-oxidant, adiabatic
UNIT-04 10L
compression, ignition source, sprays and mists, ventilation, sprinkler system, types
of explosions, explosion-proof equipment and instruments, fire and explosion
hazards, causes of fire and preventive methods.
Hazard identification and Risk Assessment: Process hazards checklists, hazard
UNIT-05 survey, hazards and operability studies (HAZOP), safety reviews, other methods, 08L
review of probability theory, event tree, fault tree, QRA and LOPA
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Develop understanding to select methods on how to prevent fires and explosions
CO2: Accomplish understanding on the effect of release of toxic substances
CO3: Acquire awareness on the methods of identification of industrial hazards and their preventive measurements
CO4: Obtained knowledge on the assessing the risk using fault tree diagram
Books and References
1. Chemical Process Safety - Fundamentals with Applications, D.A. Crowl, and J.F. Louvar, 3rd edition, Prentice
Hall, 2011.
2. Loss Prevention in Process Industries by F.P. Lees, 2nd edition, Butterworth, London, 1996.
3. Safety in Process Plant Design by G.L. Wells, George Godwin Ltd., New York, 1980.
4. Safety Health and Environmental Protection by C.A. Wentz, McGraw Hill, 2001.
Department of Chemical Engineering, NIT Hamirpur Page 37 of 56
Course Name: Optimization of Chemical Processes
Course Code: CH-430
Course Type: Professional Elective-I
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To give basics of optimization problems.
To discuss various linear and non-linear techniques.
To apply optimization techniques in chemical engineering process and design problems.
Unit Number Course Content Lectures
Introduction: Introduction to optimization and its scope in chemical process design,
Organization of optimization problems, The Essential features of optimization
UNIT-01 04L
problems, General procedure for solving optimization problems, Obstacles to
optimization.
Classical Optimization Techniques: One dimensional minimization methods:
Elimination methods- equally spaced points method, Fibonacci method and golden
UNIT-02 05L
section method; Interpolation methods-quadratic interpolation and cubic
interpolation, Newton and quasi-Newton methods.
Linear Programming and Applications: Basic concepts in linear programming,
UNIT-03 Graphical Solution, Simplex methods, Sensitivity analysis, Duality in linear 06L
programming, Transportation Problem
Multivariable Non–Linear Programming: Unconstrained-univariate method,
Powell’s method, simplex, method, rotating coordinate method, steepest descent
UNIT-04 method, Fletcher Reeves method, constrained-complex method, feasible directions 12L
method, GRG method, penalty function methods and augmented Lagrange
multiplier method.
Optimization of Staged and Discrete Processes: Dynamic programming, Integer
UNIT-05 03L
and mixed integer programming.
Application of Optimization: Heat transfer and energy conservation, Separation
UNIT-06 06L
processes, Fluid flow systems, Chemical reactor design and operation
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Formulate a chemical engineering optimization problem.
CO2: Apply suitable optimization technique to solve the problem.
CO3: Develop some numerical code for a complex optimization problem.
Books and References
1. Optimization of Chemical Processes by T.F. Edgar, D.M. Himmelblau, and L.S. Lasdon, 2nd edition, McGraw Hill,
2001.
2. Engineering Optimization Theory and Practice by S.S. Rao, 3rd edition, New Age International Publishers, 2016.
3. Engineering Optimization, Methods and Applications by A. Ravindran, K.M. Ragsdell, and G.V. Reklaitis, 2nd
edition, John Wiely, 2006.
Department of Chemical Engineering, NIT Hamirpur Page 38 of 56
Course Name: Computational Fluid Dynamics
Course Code: CH-431
Course Type: Professional Elective-I
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To develop an understanding for the major theories, approaches and methodologies used in CFD.
To impart knowledge about the application of CFD analysis to real engineering designs.
The CFD techniques can be applied for solving practical problems in fluid flow, heat and mass transfer.
To equip students with the knowledge of using commercial software package.
Unit Number Course Content Lectures
Introduction to Computational Fluid Dynamics: Introduction of CFD, Applications,
UNIT-01 comparison between numerical, analytical and experimental approaches, modeling versus 03L
experimentation.
Principles of Conservation: Fundamental principles of conservation, Reynolds transport
UNIT-02 theorem, conservation of mass, conservation of linear momentum: Navier-Stokes equation, 09L
conservation of energy, general scalar transport equation, turbulence modeling.
Classification of Partial Differential Equations: Mathematical classification of partial
UNIT-03 differential Equation, physical and mathematical classifications of PDEs, systems of partial 4L
differential equations, boundary conditions.
Finite Difference Method: Discretization principles, truncation and round-off error, explicit
and implicit approaches, basic of finite difference method, treatment of boundary conditions,
assessing accuracy and stability of numerical methods, finite difference applications in heat
UNIT-04 conduction and convection: steady and transient heat conduction in rectangular and 10L
cylindrical geometries, convective heat transfer, solution of viscous incompressible flows by
stream function-vorticity formulation solution of Navier-Stokes equation for incompressible
flows using SIMPLE algorithm
Finite Volume Method: Discretization methods, the four basic rules, one-dimensional
steady and unsteady diffusion problems, two and three dimensional situations, convection
UNIT-05 and diffusion for one-dimensional steady problems, various discretization schemes, solution 10L
of discretized equations, pressure and velocity corrections, introduction to standard -
model for turbulent incompressible flow
Course Outcomes
Upon successful completion of the course, the students will be able to
CO1: Identify usefulness of CFD over experimentation and analytical approaches and basics of conservation principles
CO2: Find type of governing partial differential equation with physical description
CO3: Learn basic principles of finite difference method and its application in physical problems
CO4: Learn basic principles of finite volume method and its application in physical problems
CO5: Develop the power of numerical solution SOFTWARE to predict complex flows
Books and References
1. An Introduction to Computational Fluid Dynamics: The Finite Volume Method by H.K. Versteeg, and W. Malalasekera,
Prentice-Hall Inc.
2. Computational Fluid Dynamics: The Basics with Applications by J.D. Anderson Jr., McGraw-Hill.
3. Computational Fluid Mechanics and Heat Transfer by D.A. Anderson, J.C. Tannehill., and R.H. Pletcher, Hemisphere
Publishing Corporation.
4. Computational Fluid Dynamics by K.A. Hoffmann, and S.T. Chiang, Engineering Education System, USA.
5. Computational Fluid Flow and Heat Transfer by K. Muralidhar, and T. Sundararajan, Narosa Publishing House, New
Delhi.
Department of Chemical Engineering, NIT Hamirpur Page 39 of 56
Course Name: Instrumental Analytical Techniques
Course Code: CH-432
Course Type: Professional Elective-I
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To impart knowledge about the statistical methods used in analyzing the data from analytical instruments.
To introduce the fundamentals of analytical instruments used in chemical industries.
To enable the student to identify the suitability of a particular analytical method(s) based on its merits, demerits,
and limitations and to interpret the output data in required form.
Unit Number Course Content Lectures
Instrumental Method of Chemical Analysis: Classification, spectroscopy,
electromagnetic spectrum, properties of electromagnetic radiation, types of
UNIT-01 molecular energies, interaction of electromagnetic radiation with matter, error, 06L
significant figure, precision, accuracy, methods of expressing accuracy, qualitative
and quantitative analysis.
Molecular Spectroscopy: Photometer, spectrophotometer, colorimeter, deviation
from Beer-Lambert law, types of transitions, theory, instrumentation, working
UNIT-02 principle, application and limitation: UV visible spectrophotometer, flame 08L
photometer, atomic absorption spectrophotometer, infrared spectroscopy, Raman
spectrophotometer, nuclear magnetic resonance and mass spectroscopy.
Morphology and Crystallography Analysis: Theory, working principals,
UNIT-03 applications, advantages and limitations: X-ray diffraction (XRD), scanning electron 08L
microscope (SEM), transmission electron microscopy (TEM).
Chromatography Techniques: Chromatography, paper chromatography, thin
layer chromatography, liquid-liquid partition chromatography, high performance
UNIT-04 08L
liquid chromatography, ion exchange chromatography, gel chromatography and
gas chromatography.
Thermal Methods: Introduction, instrumentation, advantage, application and
limitation: thermal method of analysis, thermogravimetry (TG), differential thermo
UNIT-05 06L
gravimetric (DTG), differential thermal analysis (DTA), differential scanning
calorimeter (DSC).
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Identification of the need of specific analytical method(s).
CO2: Describe and analyze the statistical methods.
CO3: Apply principles of quantitative analysis used for aqueous and solid sample characterization.
CO4: Asses the specific technique employed for characterizing different solutes in water.
Books and References
1. Principles of Instrumental Analysis by D.A. Skoog, F.J. Holler, and T.A. Nieman, 7 th edition, Cengage
Learning, 2018.
2. Instrumental Method of Chemical Analysis by G.R. Chatwal, and S.K. Anand, Himalaya Publishing House,
2005.
3. Instrumental Method of Analysis by H.H. Willard, L.L. Merritt, J.A. Dean, and F.A. Settle, 7th edition,
Wadsworth Publishing Company, 1988.
4. Chromatographic Methods by A. Braithwaite, and F.J. Smith, 5th edition, Blackie Academic and Professional,
London, 1996.
Department of Chemical Engineering, NIT Hamirpur Page 40 of 56
Course Name: Soft Computing Methods in Chemical Engineering
Course Code: CH-433
Course Type: Professional Elective-I
Contact Hours/Week: 3L Course Credits: 03
Course Objective
To apply soft computing methods in various chemical process.
Unit Number Course Content Lectures
Artificial Intelligence (AI): AI in chemical engineering, introduction to AI
UNIT-01 programming, introduction to prolog, introduction to AI principles, prolog, expert 07L
system for separation synthesis
Artificial Neural Networks (ANN): Introduction to ANN in chemical engineering,
UNIT-02 fundamentals of neural networks, application of ANN to process control, fault 07L
diagnosis, process modeling, process forecasting, limitations of ANN
Research Methodology: Knowledge based applications in chemical engineering,
UNIT-03 process fault diagnosis, process control, process planning and operation, product 07L
design and development, process modeling and simulation
Response Surface Methodology (RSM): Elementary concept of statistics,
UNIT-04 07L
significance tests, linear regression, hypothesis testing, analysis of variance
Design of Experiments: Nonlinear parameter estimation, model building and model
UNIT-05 08L
discrimination.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Understand the use of Artificial Intelligence in chemical engineering and develop the idea on knowledge based
applications in chemical engineering.
CO2: Solve engineering problems using soft computational techniques.
CO3: Convert problem solving strategies to procedural algorithms and to write program structures
CO4: Identify advanced control techniques for chemical process.
Books and References
1. Artificial Intelligence in Chemical Engineering by T.E. Quantrille, and Y.A. Liu, Academic Press, 1991.
2. Design and Analysis of Experiments by D.C. Montgomery, John Wiley and Sons, 1984.
3. Computational Methods for Process Simulation by W.F. Ramirez, Butterworth Heinemann, 1998.
4. Applied Regression Analysis by N.R. Draper, and H. Smith, Vol-1, Wiley, 1998.
5. Experimental Methods for Engineers by J.P. Holman, 7th edition, McGraw-Hill, Singapore, 2001.
6. Process Analysis by Statistical Analysis by D.M. Himmelblau, John Wiley and Sons, 1970.
7. Mathematical Modeling in Chemical Engineering by R.G. Franks, Wiley Publications, 1967.
Department of Chemical Engineering, NIT Hamirpur Page 41 of 56
Course Name: Fuel Cells and Hydrogen Energy
Course Code: CH-450
Course Type: Professional Elective-II
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To provide comprehensive and logical knowledge of hydrogen production, storage and utilization.
To learn the fundamental knowledge about various fuel cell technologies.
Unit Number Course Content Lectures
Fuel Cells: History, working principle of fuel cells, fuel cell thermodynamics, fuel
cell electrochemistry - Nernst equation, electrochemical kinetics, Butler-Volmer
UNIT-01 equation, performance evaluation of fuel cells, types of fuel cells: AFC, PAFC, 08L
SOFC, MCFC, DMFC, relative merits and demerits.
Fuel Cell Characterization: in-situ and ex-situ characterization techniques, I-V
UNIT-02 06L
curve, frequency response analyses; Fuel cell system integration
Application of Fuel Cells: Fuel Cell usage for domestic power systems,
UNIT-03 environmental analysis, large scale power generation, automobile. Future trends in 06L
fuel cells, portable fuel cells, laptops, mobiles, submarines.
Hydrogen Energy Systems: Properties of hydrogen as a fuel, hydrogen pathways,
UNIT-04 current uses, infrastructure requirement for hydrogen production, storage, dispensing 04L
and utilization, and hydrogen production plants
Hydrogen Production Processes: Thermal-steam reformation, thermo chemical
UNIT-05 water splitting, gasification-pyrolysis, nuclear thermal catalytic and partial oxidation 06L
methods. Electrochemical-Electrolysis, photo electro chemical method.
Hydrogen Storage and Safety: Physical and chemical properties, general storage
methods, compressed storage-composite cylinders, metal hydride storage, carbon
UNIT-06 based materials for hydrogen storage. Hydrogen safety aspects, backfire, pre- 06L
ignition, hydrogen emission, NOx control techniques and strategies, hydrogen
powered vehicles.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Evaluate the performance of fuel cells under different operating conditions.
CO2: Select and defend appropriate fuel cell technology for a given application.
CO3: Design and develop suitable hydrogen storage system to be used along with fuel cell system.
CO4: Minimize environmental hazards associated with the use of hydrogen storage and fuel cell technology.
Books and References
1. Electrochemical Methods by A.J. Bard, and L.R. Faulkner, 2nd edition, John Wiley & Sons, 2001.
2. Fuel Cell Fundamentals by O’Hayre, S.W. Cha, W. Colella, and F.B. Prinz, Wiley, 2005.
3. Principles of Fuel Cells by X. Li, Taylor & Francis, 2005.
4. Fuel Cell Systems Explained by J. Larminie, and A. Dicks, 2nd edition, John Wiley & Sons, 2003.
5. Fuel Cells: From Fundamentals to Applications by S. Srinivasan, Springer, 2006. €
Department of Chemical Engineering, NIT Hamirpur Page 42 of 56
Course Name: Energy Engineering
Course Code: CH-451
Course Type: Professional Elective-II
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To emphasize on the role of energy, its consumption and future demand in modernization of the world
To explain the importance of energy obtained from renewable and non-renewable resources
To develop understanding on processing and handling the solid, liquid and gas fuels
Unit Number Course Content Lectures
Introduction: Energy scene of supply and demand in India and the world, energy
UNIT-01 02L
consumption in various sectors, potential of energy resources.
Solid fuels: Coal, origin, composition, classification of coal, properties of coal, proximate
and ultimate analysis, classification of Indian coals, coal preparation, coal washing, storage
UNIT-02 06L
of coal, carbonization, liquefaction and gasification, briquetting of coal, gasification and
liquefaction of solid fuels
Gaseous fuels: Natural gas, hydrogen, methane from coal mines, producer gas, water gas,
UNIT-03 carburetted waster gas: lugri process, winkler process, koppers-totzek process, coal gas, 06L
blast furnace gas, gases from biomass, refinery gas and LPG
Solar Energy: Solar radiation and its measurement, limitations in the applications of solar
energy, solar collectors: types, and constructional details. Solar water heating, applications
UNIT-04 06L
of solar energy for heating, drying, space cooling, water desalination, solar concentrators,
photovoltaic power generation using silicon cells.
Bio-Fuels: Importance, combustion, pyrolysis and other thermo chemical processes for
biomass utilization. Alcoholic fermentation and anaerobic digestion for biogas production.
UNIT-05 08L
Wind Power: Principle of energy from wind, windmill construction, operational details,
electricity generation and mechanical power production.
Tidal Power: It’s meaning, causes of tides and their energy potential, enhancement of tides,
power generation from tides and problems. Principles of ocean thermal energy conversion
(OTEC) analysis and sizing of heat exchangers for OTEC.
UNIT-06 Geothermal Energy: Geo technical wells and other resources dry rock and hot aquifer 08L
analysis, harnessing geothermal energy resources. Energy Storage and Distribution:
Importance, biochemical, chemical, thermal, electric storage. Fuel cells, distribution of
energy.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Estimate the energy demand worldwide and available resources to overcome future demand
CO2: Understand about the conventional energy sources like solid and gaseous fuels
CO3: Understand about the non-conventional energy sources like solar energy, bio-fuels, wind power, tidal power and
geothermal energy.
Books and References
1. Fuels and Combustion by S. Sarkar, Universities Press, 3rd edition, 2009.
2. Non-Conventional Energy Sources by G.D. Rai, Khanna Publishers, New Delhi, 2001.
3. Fuels- Solid, Liquid and Gaseous by J.S. Brame, and J.C. King, St. Martin Press.
4. Renewable Energy by B. Sorenson, 3rd edition, Elsevier Science, 2004.
5. Renewable Energy Resources by J. Twiddle, and T. Weir, Cambridge University Press, 1986.
6. Solar Energy: Principles of Thermal Collection & Storage by S.P. Sukhatme, 2nd edition, Tata McGraw-Hill, 2001.
7. Solar Energy: Fundamentals and Applications by H.P. Garg, and J. Prakash, Tata McGraw-Hill, 2001.
Department of Chemical Engineering, NIT Hamirpur Page 43 of 56
Course Name: Solid Waste Management
Course Code: CH-452
Course Type: Professional Elective-II
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To facilitate understanding of issues and approaches associated with solid waste, hazardous waste and special
category waste management.
To enable the students to access legal requirements and strategies associated with management of municipal,
hazardous and special solid waste.
Unit Number Course Content Lectures
Characterization and Quantification: Solid waste management, nuisance potential
UNIT-01 and extent of solid waste problems, regulatory requirements, types and composition 08L
of solid waste, methods of quantification and characterization of wastes.
Collection, Storage and Transportation of Wastes: Types of collection systems
UNIT-02 and their components, segregation at source, solid waste transport vehicles, solid 06L
waste transit points and transport routes, storage and handling of hazardous waste.
Municipal Solid Waste Management: Recycling, recovery of useful components of
UNIT-03 solid waste and its applications, composting, bio-gasification, waste to energy 06L
production.
Hazardous waste Management: Definition, sources, classification, collection and
segregation, chemical and biological treatment of hazardous waste, solidification and
UNIT-04 06L
stabilization refuse derived fuel, gasification, pyrolysis, incineration, disposal,
management of effluent treatment plant sludge.
Sanitary Landfills: Site selection and approval, design, development, operation and
UNIT-05 closer of landfills, management of leachate and landfill gases, environmental 06L
monitoring of landfill sites.
Special Category Wastes and Their Management: Construction and demolition
UNIT-06 wastes, biomedical wastes, radioactive waste, e-waste, plastic waste, oil sludge and 04L
slurries.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Understanding and appreciating the environmental pollution and nuisance potential of municipal solid waste and
of special category wastes.
CO2: Awareness of management of MSW and hazardous waste according their characteristics (selection of
management technique).
CO3: Acquiring the knowledge of collection and transportation and solid waste route selection and types of waste
collection.
CO4: Regulatory requirement applicable to the handling and management of MSW and special category waste.
Books and References
1. Waste management Practices-Municipal, Hazardous and Industrial by J. Pichtel, CRC Press, 2005.
2. Solid Waste Engineering by P.A. Vesilind, Thomson, 2008.
3. United Nations Environment Programme (UNEP) Solid Waste Management, 2005.
4. Solid Waste Management in Developing Countries by A.D. Blude, and B.B. Sudaresan, INSDOC, 1972.
5. Integrated Solid Waste Management Engineering Principles and Management Issues, G. Tchobanoglous, H.
Theisen, and S.A. Vigil, McGraw Hill, 1993.
6. Hazardous Waste Management by M.D. LaGrega, P.L. Buckingham, and J.C. Evans, Waveland Press, 2010.
Department of Chemical Engineering, NIT Hamirpur Page 44 of 56
Course Name: Reservoir Engineering
Course Code: CH-453
Course Type: Professional Elective-II
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To understand a petroleum reservoir and study the basic properties of rock and reservoir fluid.
To enable the students to identify the concept of PVT analysis and material balance of hydrocarbon reservoir to
estimate the oil in place.
To visualize the fluid flow, understand the drive mechanism and various methods for oil and gas recovery.
Unit Number Course Content Lectures
Fundamentals of Reservoir Engineering: Origin and composition of petroleum,
petroleum geology, calculation of hydrocarbon volumes, fluid pressure regime, oil
UNIT-01 recovery: recovery factor, volume gas reservoir engineering, application of the real 06L
gas equation of state, gas material balance: recovery factor, hydrocarbon phase
behavior, PVT analysis
Reservoir Rock and Fluid Properties: Porosity, saturation, wettability, surface and
interfacial tension, capillary pressure, permeability, rock compressibility, net pay
UNIT-02 06L
thickness, reservoir heterogeneity, areal heterogeneity, two and three phase relative
permeability, drainage and imbibition process
Material Balance in Oil and Gas Reservoirs: General material balance for
hydrocarbon reservoir, material balance expressed as linear equation, reservoir drive
UNIT-03 08L
mechanisms, solution gas drive, gas cap drive, natural water drive, compact drive
and combination drive
Flow in Porous Media: Types of fluid, flow regime, reservoir geometry, number of
flowing fluid in the reservoir: Darcy’s Law, steady and unsteady state flow, skin
UNIT-04 factor, radial steady state flow: well simulation, two phase flow: effective and relative 08L
permeability, derivation of the basic radial differential equation, conditions of
solutions, theory of well testing
Enhance Oil Recovery Techniques: Basic principles and mechanism of EOR, IOR
and EOR, selection criteria for EOR, microscopic and macroscopic displacement
UNIT-05 08L
efficiency, mobility ratio, water flooding, chemical flooding, microbial and thermal
enhanced oil recovery
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: To estimate the hydrocarbon quantity by oil and gas material balance equations
CO2: Acquire the knowledge on multiphase flow and flow of fluid in porous media
CO3: Visualize the complexities found in reservoir and various method available to enhance maximum cumulative oil
recovery
CO4: Convert them into a successful reservoir engineer for oil and gas industry
Books and References
1. Fundamentals of Reservoir Engineering by L.P. Dake, Elsevier, 1983.
2. Enhanced Oil Recovery, D.W. Green, and G.P. Willhite, SPE Textbook Series, Vol. – 6, 1998.
3. Applied Petroleum Reservoir Engineering by R.E. Terry, M. Hawkins, and B.C. Craft, Prentice Hall, 1991.
4. Reservoir Engineering Handbook by T. Ahmed, 3rd edition, 2006.
Department of Chemical Engineering, NIT Hamirpur Page 45 of 56
Course Name: Biochemical Engineering
Course Code: CH-440
Course Type: Professional Elective-III
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To impart the basic importance and need for biochemical engineering in industry.
To develop understanding about enzyme and cell kinetics.
To enable the students to understand the various aspects of bioreactor design.
Unit Number Course Content Lectures
Introduction to Bioscience: Types of microorganisms, structure and function of
microbial cells, fundamentals of microbial growth, batch and continuous culture,
UNIT-01 sterilization methods, isolation and purification of enzymes from cells. Downstream 08L
processing and product recovery in bioprocesses, assay of enzymes, cell growth
measurement.
Enzyme Technology and Kinetics: Applied enzyme catalysis, immobilization of
UNIT-02 enzymes, kinetics of enzyme catalytic reactions involving isolated enzymes, enzyme 07L
inhibition, effect of pH and temperature on enzyme activity
Enzymatic Reactions and reactor analysis: Reactor design and analysis for
soluble enzyme systems. Cofactor regeneration, membrane reactor, effect of mass
UNIT-03 07L
transfer in immobilized enzyme particle systems, reactors for immobilized enzyme
systems.
Transport processes in Bio reactors and its design: Batch, fed-batch and
continuously stirred aerated tank bioreactors. Mixing power correlation.
UNIT-04 Determination of volumetric mass transfer rate of oxygen from air bubbles and effect 08L
of mechanical mixing and aeration on oxygen transfer rate, heat transfer and power
consumption. Multiphase bioreactors and their applications.
Applications: Applications of enzymes in industry and medicine, carbohydrates,
UNIT-05 starch conversion and cellulose conversion, Food industry, Biological wastewater 06L
treatment.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Gain general knowledge about cell cultivation and enzymatic processes and downstream processing.
CO2: Get a working knowledge of different immobilization methods and enzyme inhibitions.
CO3: Understand enzyme kinetics and cell kinetics.
CO4: Design various bioreactors.
Books and References
1. Biochemical Engineering Fundamentals by J.E. Bailey, and D.F. Olis, McGraw-Hill, 1987.
2. Bioprocess Engineering by M.L. Schuler, and F. Kargi, Prentice Hall, 2002.
3. Bioprocess Engineering Principles by P.M. Doran, Academic Press, 2013.
4. Biochemical Engineering by M. Doble, and S.N. Gummadi, Prentice Hall, 2007.
5. Chemical Engineering by J.M. Coulson, and R.E. Richardson, Vol. - 3, Elsevier, 2014.
Department of Chemical Engineering, NIT Hamirpur Page 46 of 56
Course Name: Food Science and Engineering
Course Code: CH-441
Course Type: Professional Elective-III
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To impart knowledge to the students about food process engineering
To teach about food preservation and packaging.
To understand the hazards and safety in food industries.
Unit Number Course Content Lectures
Introduction: General aspects of food industry, composition of foods, quality and
UNIT-01 nutritive aspects, characteristic features of processed and natural food, mass and 06L
energy balance in food processing operation.
Food Rheology: Characteristics of non-Newtonian fluids, time-independent and
UNIT-02 06L
time dependent non-Newtonian fluids, linear viscoelastic fluids.
Thermal Processing: Canning/retort processing – process design and
equipment’s. Equipment design aspects of pasteurizer, sterilizers, evaporators and
UNIT-03 07L
concentrators, dryers and their design parameters – tray dryer, spray dryer,
fluidized bed dryer.
Food Preservation: Microbial survivor curves, thermal death of microorganisms
and D, Z and F value calculation, spoilage probability, food preservation by
UNIT-04 06L
dehydration, irradiation, Food preservation by adding preservatives. Food
production,
Packaging and Storage: Process design aspects for liquid foods such as milk and
juices. Concentration with thermal and membranes processes. Food packaging and
UNIT-05 07L
product shelf life, modified atmosphere and controlled atmosphere storage, aseptic
packaging, freezing and thawing calculations.
UNIT-06 Food Laws: Legislation, safety and quality control. 04 L
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Understand rheological properties of foods.
CO2: Identify and evaluate various design parameters for thermal equipment for food.
CO3: Quantify thermal death of micro-organism and calculate spoilage probability
CO4: Evaluate effect of food processing and packaging/storage on food quality
CO5: Analyze food related hazards and HACCP method.
Books and References
1. Food Science by N.N. Potter, and H. Joseph, CBS Publisher, 2005.
2. Fundamentals of Food Process Engineering by T. Romeo, CBS Publisher, 2007.
3. Food Processing by V.H. Potty, and M.J. Mulky, Oxford and IBH, 1993.
4. Food Process Engineering by D.R. Heldman, and R.P. Singh, Chapman and Hall, 1984.
5. Food Microbiology by W.C. Frazier, Tata McGraw Hill, 2007.
Department of Chemical Engineering, NIT Hamirpur Page 47 of 56
Course Name: Fertilizer Technology
Course Code: CH-442
Course Type: Professional Elective-III
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To impart knowledge about N-P-K fertilizers and calculation of N-P-K values in complex fertilizers.
To introduce the fundamentals of fertilizer preparation technology and respective flow diagrams.
To enable the student to understand various problems occurring in fertilizer production and dealing with these
problems as a chemical engineer.
Unit Number Course Content Lectures
Introduction: Elements required for plants growth, classification of fertilizers,
UNIT-01 08L
compound, complex and bulk blended fertilizers. N-P-K values and calculations.
Nitrogenous Fertilizers: Manufacturing processes for ammonia, effects of various
factors on the process. Manufacture of ammonium sulphate, ammonium chloride,
UNIT-02 10L
ammonium phosphate, ammonium nitrate, nitric acid, urea, etc. Economics and
other strategies, material of construction and corrosion problem.
Phosphatic Fertilizers: Calculation of percentage tri-calcium phosphate of lime in
UNIT-03 phosphatic rock. Manufacture of single triple super phosphate. Nitrophosphate, 10L
sodium phosphate, phosphoric acid and other phosphatic fertilizers.
Potash Fertilizers: Manufacture of potash fertilizers like potassium sulphate,
UNIT-04 08L
potassium chloride, etc.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Identification and calculation of complex fertilizers.
CO2: Describe and analyze the production methods of nitrogenous, phosphatic, and potash fertilizers using simple
process flow diagram and complex process and instrumentation diagram.
CO3: Apply principles of chemical engineering in fertilizer production like mass and energy balances.
CO4: Asses the problems in production process and then addressing problems by using chemical engineering
basics.
Books and References
1. Commercial Fertilizers by G.H. Collings, 5th edition, McGraw Hill, New York, 1955.
2. Outlines of Chemical Technology by C.E. Dryden, East-West Press, New Delhi, 1973.
3. A Text Book of Chemical Technology by S.D. Shukla, and G.N. Pandey, Vol.-2, Vikas Publishing House, New
Delhi.
4. Chemistry and Technology of Fertilizers by A.V. Slacks, Interscience, New York, 1966.
Department of Chemical Engineering, NIT Hamirpur Page 48 of 56
Course Name: Novel Separation Processes
Course Code: CH-443
Course Type: Professional Elective-III
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To understand fundamentals of novel separation processes
To study various membrane separation processes
To study separation of biochemicals
To understand basics of chromatography
Unit Number Course Content Lectures
Fundamentals of Separation Processes and Membrane Separation Processes:
Mechanisms of phase separation, selection of feasible separation process, various
novel separation processes, principles of membrane separation, classification, analysis
UNIT-01 and modeling of membrane separation, osmosis, nanofiltration, microfiltration and 10L
ultrafiltration, membrane characteristics and applications, ion selective membranes and
their application in electrolysis, dialysis and electro dialysis, pervaporation and gas
separation
Surfactant Based Separation Processes: Foam and bubble separation, principle,
UNIT-02 06L
classification, foam and surfactants, separation techniques, column separations
Centrifugal Separation Processes: Principle, settling rates, sigma value and scale-up
UNIT-03 05L
issue, separation of liquids
Electrophoretic Separation Methods: Forces in electrophoresis, factors influencing
UNIT-04 05L
electrophoresis, gel membrane and paper electrophoresis, zonal electrophoresis
Supercritical Fluid Extraction (SCF): Critical conditions, supercritical solvents,
UNIT-05 05L
parameters in SCF, basic technique.
Ion Exchange and Chromatographic Separation Processes: Principles and practice,
classification of chromatographic techniques, gel filtration, ion exchange
UNIT-06 05L
chromatography and chromato focusing, reversed phase and hydrophobic interaction
chromatography, affinity chromatography
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Select a suitable separation process for a desired application.
CO2: Design the separation process selected.
CO3: Can develop hybrid systems for more advanced separation.
CO4: Can apply separation techniques to biological systems.
Textbooks:
1. Membrane Separation Processes by K. Nath, PHI, 2012.
2. Separation Process Principles by J.D. Seader, E.J. Henley, and D.K. Roper, John Wiley, 2015.
3. Transport Phenomena and Separation Process Principles by J. Genkoplis, PHI, 2015.
4. Separation Processes by C.J. King, McGraw Hill, 2013.
5. Water Purification by Ion Exchange by T.V. Arden, Springer, 1968.
6. Unit Operations of Chemical Engineering by W.L. McCabe, J.C. Smith, and P. Harriott, McGraw Hill, 2017.
7. Handbook of Separation Process Technology by R.W. Rousseau, Wiley-Blackwell, 1987.
Department of Chemical Engineering, NIT Hamirpur Page 49 of 56
Course Name: Polymer Science and Engineering
Course Code: CH-460
Course Type: Professional Elective-IV
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To impart knowledge about industrial manufacturing processes.
To introduce the fundamental knowledge about polymerization reaction kinetics.
To enable the students about technology and application of commodity of plastics.
Unit Number Course Content Lectures
Introduction: Concepts and classification of polymers Functionality, Glass transition
temperature, Addition, condensation, step- growth and chain –growth polymerization.
UNIT-01 Molecular weight estimation: Average molecular weight – number and weight 09L
average, Sedimentation and viscosity average molecular weights, Molecular weight
and degree of polymerization, poly dispersity index, significance of molecular weight.
Polymerization Processes: Bulk, solution, emulsion and suspension polymerization,
UNIT-02 05L
Comparison of polymerization processes.
Polymerization Kinetics: Chemistry of step reaction polymerization, mechanism and
kinetics of polycondensation reactions and free-radical chain polymerization, chain
UNIT-03 07L
transfer agents, Ziegler Natta polymerization processes and differentiation based on
kinetics of anionic and cationic polymers.
Synthetic Fibres: Types of fibres, spinning techniques, manufacturing technology
UNIT-04 and applications of different types of fibres: cellulosic fibres, polyamides, acrylics, 07L
vinyls and vinylidines, fluorocarbons.
Plastics: Molding techniques for plastics: injection molding, compression molding,
calendaring, blow moulding, extrusion, thermoforming, and applications of different
UNIT-05 types of plastics: polyester, polyethylene, phenolics, rubbers, structure, properties and 08L
preparation natural rubber synthetic rubbers: SBR, rubber compounding and
reclaiming.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Able to understand the basic classification of polymers.
CO2: Analyze the various techniques of carrying out polymerization
CO3: Determine the molecular weight of polymers
CO4: Describes the various polymer processing techniques
Books and References
1. Polymer Science by V.R. Gowariker, N.V. Viswanathan, and J. Sreedhar, New Age International Publishers,
1996.
2. Text Book of Polymer Science by F.W. Billmeyer, Wiley Tappers, 1994.
3. Polymer Science and Technology of Plastics and Rubber by P. Ghosh, Tata McGraw Hill, 2001.
4. Fundamentals of Polymer Engineering by R.K. Gupta, and A. Kumar, 2nd edition, Marcel Dekkar, 2003.
Department of Chemical Engineering, NIT Hamirpur Page 50 of 56
Course Name: Colloid and Interface Science
Course Code: CH-461
Course Type: Professional Elective-IV
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To familiarize with the knowledge about nano and micro scale interfacial phenomena.
To provide fundamental concepts of difference between bulk and micro fluidics.
To equip students with the knowledge of stability theory of thin film flows.
Unit Number Course Content Lectures
Fluid Interfaces and Capillarity: Concepts of surface and interfacial energies and
tensions, apolar (Van der Waals) and polar (acid-base) components of interfacial
UNIT-01 tension, Young-Laplace equation of capillarity, Kelvin equation, stability of equilibrium 8L
solutions, contact angle and Young’s equation, thin liquid films.
Thermodynamics of Interfacial Systems: Thermodynamics concepts, capillary
system, Gibb’s treatment of interfaces, concept of excess concentration, variation of
UNIT-02 06L
interfacial tensions with surfactant concentration, self-assembly of surfactants, insoluble
monomers, adhesion, wetting, flotation.
Electrical Phenomena at Interfaces: Electrostatic double layer, zeta potential, acid-
UNIT-03 base interactions including hydrophobic attraction and hydration pressure, 6L
electrokinetics.
Interaction Between Colloid Particles: Long range van der Waals interactions,
UNIT-04 8L
electrostatic interactions, DLVO theory, and kinetics of flocculation.
Interfacial Hydrodynamics: Unbalanced forces at fluid interfaces, Marangoni effect
UNIT-05 8L
and its practical implications, effect of surfactants, Gibb’s elasticity.
Course Outcomes
Upon successful completion of the course, the students will be able to
CO1: Understand basic concepts and tools of colloid and interface science and engineering
CO2: Understand of differences between the surface and bulk dominated regimes and behavior and exploitation of
nano-behavior.
CO3: Understand the stability concepts of micro-scale fluid flows.
Books and References
1. Principles of Colloid and Surface Chemistry by P.C. Hiemenz, and R. Rajagopalan, Marcel Dekker, New York,
1997.
2. An Introduction to Interfaces and Colloids: The Bridge to Nanoscience by J.C. Berg, World Scientific Singapore,
2010.
3. Intermolecular and Surface Forces by J. Israelachvili, Academic Press, New York, 1992.
4. Physical Chemistry of Surfaces by A.W. Adamson, and A.P. Gast, John Wiley & Sons, New York, 1997.
Department of Chemical Engineering, NIT Hamirpur Page 51 of 56
Course Name: Nanomaterials and Nanofabrication
Course Code: CH-462
Course Type: Professional Elective-IV
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To impart knowledge about the basic concepts of nanomaterials and nanotechnology.
To introduce the fundamental concepts relevant to different classes of nanomaterials.
To enable the students to understand the factors that causes the design and fabrication of nanoparticles.
Unit Number Course Content Lectures
Introduction: Definition of nanotechnology and nanomaterials, nanoscale dimension,
history, current issues and industry applications, different classes of nanomaterials as
UNIT-01 metal and semiconductor nanomaterials, quantum dots, wells and wires, bucky balls 06L
and carbon nanotubes, self-assembly, complex adaptive systems (CAS), carbon
nanomaterials.
Synthesis Techniques: Top-down approach, bottom-up approach, grinding, planetary
milling and comparison of particles, sol-gel methods, sonochemical approach, physical
UNIT-02 07L
vapour deposition, chemical vapour deposition, wet deposition techniques,
supramolecular approach, molecular design and modelling.
Characterization Techniques: Instrumentation fractionation principles of particle size
UNIT-03 measurements, particle size and its distribution, XRD, TEM, SEM and AFM technique, 06L
scanning and tunnelling microscopy, fluorescence microscopy and imaging.
Carbon Nanotubes: Introduction to carbon nanotube, CNT from graphite, types of
UNIT-04 05L
CNT, nanotubes and nano-wall structures, bucky onions nanotubes.
Nanofabrication: Nanolithography, photolithography, soft lithography, thin film
UNIT-05 deposition, etching and bonding, micro-electro-mechanical systems (MEMS), 05L
challenges & future development.
Industrial Applications: Solar energy conversion and catalysis, molecular electronics
and printed electronics, liquid crystalline systems, applications in displays and other
devices, advanced organic materials for data storage, photonics, plasmonics, chemical
UNIT-06 07L
and biosensors, nanomedicine and nano-biotechnology, applications in drug delivery,
coating, membrane based application, polymer and paints industry, food and agriculture
Industry, cosmetics, water treatment.
Course Outcomes
Upon Successful completion of the course, the students will be able to
CO1: Identify and understand the peculiar properties of materials at nanoscale.
CO2: Describe the chemistry involved in the synthesis and fabrication of nanomaterials.
CO3: Apply principle of nanotechnology to understand the properties of nanomaterials.
CO4: Assess the importance of applications of nanomaterials in related fields.
Books and References
1. Introduction to Nanoscience and Nanotechnology by G.L. Hornyak, H.F. Tibbals, J. Dutta, and J.J. Moore, CRC
Press, 2009.
2. Introduction to Nanotechnology by C. Poole, and F. Owens, Wiley India, 2007.
3. Nanoscale Science and Technology by R. Kelsall, I.M. Hamley, and M. Geoghegan, John Wiley, 2005.
4. NANO: The Essentials: Understanding Nanoscience and Nanotechnology by T. Pradeep, McGraw Hill, 2007.
Department of Chemical Engineering, NIT Hamirpur Page 52 of 56
Course Name: Heterogeneous Catalysis and Catalytic Processes
Course Code: CH-463
Course Type: Professional Elective-IV
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To gain the knowledge of catalyst characteristics
To impart the knowledge about mechanism of catalytic reactions
To enable the students to design of catalytic reactors.
Unit Number Course Content Lectures
Catalysis: Homogeneous and heterogeneous catalysts, classification of catalytic reactions
UNIT-01 04L
and catalysts, commercial chemical catalysts, steps in catalytic reactions.
Preparation and Properties of Catalysts: Methods of catalyst preparation, physical
properties of catalyst – surface area, pore volume, pore size distribution, solid density,
UNIT-02 08L
particle density, bulk density, void volume, catalyst promoters and inhibitors, catalyst
accelerators and poisons.
Adsorption and Catalytic Reactions: Adsorption isotherms, surface reaction, single site
UNIT-03 and dual site mechanism, desorption, catalyst deactivation, pore structure and surface area 08L
estimation and their significance.
External Transport Processes: Fluid particle mass and heat transfer, Mass transfer-limited
reactions in packed beds, Non-isothermal behavior of packed-bed reactors, Staged packed
UNIT-04 bed reactors for approaching optimum temperature progression, Stable operating conditions 08L
in reactors and hot spot formation, Effect of external transport processes on selectivity under
non-isothermal conditions.
Diffusion and Reaction in Porous Catalysts: Intra-pellet mass transfer and diffusion in
cylindrical and spherical porous catalyst particles, Thiele modulus, Diffusion controlled and
UNIT-05 surface reaction controlled kinetics, Effectiveness factor for catalysts, Effects of heat transfer 06L
– temperature gradients across fluid-solid film and across catalyst pellet, Fluidized bed
reactors, Three phase reactors – slurry and trickle bed reactors.
Generalized Design: Design of catalytic reactors under adiabatic and non-adiabatic
UNIT-06 04L
conditions, Design of industrial fixed-bed, fluidized-bed and slurry reactors.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Develop various catalytic reaction mechanisms.
CO2: Characterize a catalyst.
CO3: Assess the effects of external heat and mass transfer effects in heterogeneous catalysis.
CO4: Calculate the effectiveness of a porous catalyst.
CO5: Design different types of reactors for catalytic reactions.
Books and References
1. Chemical Engineering Kinetics by J.M. Smith, McGraw-Hill, 1981.
2. Elements of Chemical Reaction Engineering by H.S. Fogler, Prentice-Hall of India, 2009.
3. Chemical Reactor Theory: An Introduction by K.G. Denbigh, and J.C.R. Turner, Cambridge University Press, 1984.
4. Chemical and Catalytic Reaction Engineering by J.J. Carberry, McGraw-Hill, 2001.
5. Chemical Reaction Engineering by O. Levenspiel, John Wiley, 2006.
Department of Chemical Engineering, NIT Hamirpur Page 53 of 56
Course Name: Energy and Environmental Engineering
Course Code: CH-306
Course Type: Open Elective-I / II
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To inculcate fundamental knowledge and understanding of the major energy conversion processes.
To impart the knowledge about the resource requirement and their impacts on environment.
Unit Number Course Content Lectures
Introduction: Interrelationship between energy and environment, the need of sustainability,
nature & issues; environment conservation and management as the key requirements of
UNIT-01 sustainability, scope and importance, need for public awareness, energy chain and common 08L
forms of usable energy, classification of energy sources, present energy scenario, world
energy status, energy scenario in India.
Conventional Energy: Environmental impacts related to harnessing to fossil fuels (coal, oil,
UNIT-02 natural gas), nuclear energy, hydropower (overview of micro mini and small hydro power, 06L
classification of hydropower schemes), impact of energy production on climate change.
Renewable Sources of Energy: Solar energy; active and passive systems, measurement
and applications including solar water heating, solar cooking, solar drying, solar distillation
and solar refrigeration, heating and cooling of buildings, solar thermal power generation,
solar photo-voltaic power generation, process economics and environmental impacts,
biomass energy; generation, characterization, biogas (aerobic and anaerobic bio-
UNIT-03 14L
conversion processes), properties of biogas, waste to energy (domestic sewage, municipal
solid wastes); biorefineries, biohydrogen production, environmental aspects of biofuel
utilization - techno‐economic features of bio‐fuels, wind energy, wind diesel hybrid systems,
control of hybrid power systems, power generation through OTEC systems - various types -
energy through waves and tides - energy generation through geothermal systems – types.
Social Issues and the Environment: Environmental degradation, environment ethics,
UNIT-04 issues and possible solutions, urban problems related to energy, water conservation, rain 08L
water harvesting, water shed management.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Describe basic energy concepts.
CO2: Analyze the consequences of today’s energy consumption.
CO3: Account for conventional energy technologies and the relationship between energy production, consumption and
climate change.
CO4: Reflect and evaluate the environmental impact of energy production through renewable sources of energy.
Books and References
1. Energy and the Environment by R.A. Ristinen, and J.J. Kraushaar, 2nd edition, 1998.
2. Fundamentals of Renewable Energy Sources by G.N. Tiwari, and M.K. Ghosal, Narosa Publishing House, New Delhi, 2007.
3. Energy Technology by S. Rao, and B.B. Parulekar, 4th edition, Khanna Publishers, 2005.
4. Energy Science: Principles, Technologies and Impacts by J. Andrews, and N. Jelley, Oxford Universities Press, 2013.
5. Renewable Energy, Power for a Sustainable Future by G. Boyle, Oxford University Press, 2012.
6. Renewable Energy Systems, Advanced Conversion Technologies and Applications by L.Y. Fang, and Y. Hong, CRC Press, 2012.
Department of Chemical Engineering, NIT Hamirpur Page 54 of 56
Course Name: Nanoscience and Nanotechnology
Course Code: CH-370
Course Type: Open Elective-I
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To impart knowledge about the basic concepts of nanoscience and nanotechnology.
To introduce the fundamental concepts relevant to different classes of nanomaterials.
To enable the students to understand the factors that causes the design and fabrication of nanoparticles.
Unit Number Course Content Lectures
Nanoscience: Introduction and importance, definition of nano, atomic structure and size,
emergence and challenges of nanoscience, formation of CNT to Graphene, influence of nano
UNIT-01 over micro and macro, size effects, surface effects on the properties. 06L
Nanostructure and Nanomaterials Properties: Types of nanostructure and properties of
nanomaterials, one dimensional, two dimensional and three dimensional nanostructured
UNIT-02 materials, quantum dots shell structures, semiconductors, composites, mechanical-physical- 07L
chemical properties.
Nanotechnology: Introduction, emergence and challenges of nanotechnology, synthesis,
vapor condensation methods, sputtering, laser method, spray pyrolysis, thermo chemical,
UNIT-03 flame decomposition of metals, organic precursors methods. 05L
Characterization Tools: X-Ray diffraction (XRD), Scanning electron microscopy, transmission
UNIT-04 electron microscopy, atomic force microscopy, UV spectroscopy. 06L
Classification and Fabrication: Introduction and classification, electronic properties of atoms
and solids, nanometer length scale effects, fabrication methods; top down and bottom up
UNIT-05 fabrication approach, self-assembly, bio-mediated assembly, safety and storage issues. 07L
Industrial Applications: Coating, cosmetics, nano sensor, nano catalysts, water treatment,
UNIT-06 paints industry, food and agriculture Industry, biological and environmental applications. 05L
Course Outcomes
Upon Successful completion of the course, the students will be able to
CO1: Identify and understand the peculiar properties of nano-materials at nanoscale.
CO2: Describe the chemistry involved in the synthesis and fabrication of nanomaterials.
CO3: Apply principle of nanoscience and nanotechnology to understand the properties of nanomaterials.
CO4: Assess the importance of applications of nanomaterials in related fields.
Books and References
1. Introduction to Nanoscience and Nanotechnology by G.L. Hornyak, H.F. Tibbals, J. Dutta, and J.J. Moore, CRC Press,
2009.
2. Introduction to Nanotechnology by C. Poole, and F. Owens, Wiley India, 2007.
3. Nanoscale Science and Technology by R. Kelsall, I.M. Hamley, and M. Geoghegan, John Wiley, 2005.
4. NANO: The Essentials: Understanding Nanoscience and Nanotechnology by T. Pradeep, McGraw Hill, 200.
Department of Chemical Engineering, NIT Hamirpur Page 55 of 56
Course Name: Industrial Safety and Hazard Management
Course Code: CH-380
Course Type: Open Elective-II
Contact Hours/Week: 3L Course Credits: 03
Course Objectives
To impart knowledge about various aspects of industrial safety and occupational health.
To enable the students to identify hazard and assess risk.
To teach about various safety acts and rules along with safety education and training.
Unit Number Course Content Lectures
Concepts and Techniques: History of safety movement –Evolution of modern
safety concept - Incident Recall Technique (IRT), disaster control, safety analysis,
safety survey, safety inspection, safety sampling. Safety Audits-components of
UNIT-01 08L
safety audit, types of audit, audit methodology, non-conformity reporting (NCR), audit
checklist- identification of unsafe acts of workers and unsafe conditions in the
industry.
Occupational Health and Toxicology: Concept and spectrum of health, functional
units and activities of occupational health services, occupational related diseases
UNIT-02 08L
and levels of prevention of diseases. Toxicology- local, systemic and chronic effects,
temporary and cumulative effects, carcinogens entry into human systems.
Hazard Identification and Risk Assessment: The process of risk management,
UNIT-03 hazard identification, evaluation (risk assessment, risk matrix), risk control 06L
implementation, action and recommendation.
Acts and Rules: Indian boiler Act 1923, static and mobile pressure vessel rules
(SMPV), motor vehicle rules, mines act 1952, workman compensation act, rules–
electricity act and rules–hazardous wastes (management and handling) rules, 1989,
UNIT-04 07L
with amendments in 2000- the building and other construction workers act 1996.,
Petroleum rules, Explosives Act 1983-Pesticides Act, Factories Act 1948, Air Act
1981 and Water Act 1974.
Safety Education and Training: Importance of training-identification of training
needs-training methods – programmes, seminars, conferences, competitions –
method of promoting safe practice - motivation – communication - role of government
UNIT-05 07L
agencies and private consulting agencies in safety training – creating awareness,
awards, celebrations, safety posters, safety displays, safety pledge, safety incentive
scheme, safety campaign – domestic Safety and Training.
Course Outcomes
Upon successful completion of the course, the student will be able to
CO1: Identify the key aspects of industrial safety and mitigating them
CO2: Describe various types of solution to problems arising in safety operations and hygiene.
CO3: Apply principles of OSHA in controlling industrial disasters and losses
CO4: Assess the overall performance of safety protocols of chemical industries and hazard management.
Books and References
1. Industrial Accident Prevention by H.W. Heinrich, McGraw-Hill, 1980.
2. Safety Management in Industry by N.V. Krishnan, Jaico Publishing House, Bombay, 1997.
3. Loss Prevention in Process Industries by F.P. Lees, Butterworth, London, 1990.
4. Safety at Work by J.R. Ridley, Butterworth, London, 1983.
5. Chemical Process Safety Fundamentals with Applications by D.A. Crowl, and J.F. Louvar, Prentice Hall, 2002.
Department of Chemical Engineering, NIT Hamirpur Page 56 of 56
You might also like
- MG2 Series ManualDocument138 pagesMG2 Series ManualbenmedNo ratings yet
- Chemical Engineering SyllabusDocument49 pagesChemical Engineering Syllabusyugal sharmaNo ratings yet
- Syllabus FinalYr PDFDocument63 pagesSyllabus FinalYr PDFHimanshu DiwakarNo ratings yet
- B.tech - Chemical EngineeringDocument68 pagesB.tech - Chemical EngineeringAnuja ElangovanNo ratings yet
- Chemical Engineering Course Outlines - Effective From Fall 2013Document45 pagesChemical Engineering Course Outlines - Effective From Fall 2013Khan AaghaNo ratings yet
- B.Tech. Chemical Engineering PDFDocument70 pagesB.Tech. Chemical Engineering PDFJogi BogiNo ratings yet
- Syllabus PDFDocument75 pagesSyllabus PDFTehMarianNo ratings yet
- Minor New CurrDocument20 pagesMinor New CurrAnanyaNo ratings yet
- R-16 Syllabus For Ptrochemical EngineeringDocument176 pagesR-16 Syllabus For Ptrochemical EngineeringsrinuNo ratings yet
- Syllabi FEDocument32 pagesSyllabi FEfarkhandasaadat67No ratings yet
- BSC Chemical Course OutlineDocument3 pagesBSC Chemical Course Outlineniikwabena36No ratings yet
- M. TECH Thermal Engg. FT 2009Document32 pagesM. TECH Thermal Engg. FT 2009vankasrividyaNo ratings yet
- 1623398808revised BSC EngineeringDocument142 pages1623398808revised BSC EngineeringTalha MughalNo ratings yet
- Updated BTech CurriculumDocument6 pagesUpdated BTech CurriculumANKUR MESHRAMNo ratings yet
- BATU Petrochemical Syllabus PDFDocument74 pagesBATU Petrochemical Syllabus PDFashwin100% (1)
- Revised Scheme of Courses and Syllabi 4 Years (8-Semesters) B.Tech. Degree Programme in Chemical EngineeringDocument34 pagesRevised Scheme of Courses and Syllabi 4 Years (8-Semesters) B.Tech. Degree Programme in Chemical EngineeringGarima JainNo ratings yet
- B.tech. Chemical EngineeringDocument56 pagesB.tech. Chemical EngineeringpkarambeleNo ratings yet
- BTech 2022 Onwards 91043Document127 pagesBTech 2022 Onwards 91043jatinb.cm.22No ratings yet
- Ju 090818103344 0Document158 pagesJu 090818103344 0Sumit KajlaNo ratings yet
- Petrochemical Engineering R16-SyllabusDocument175 pagesPetrochemical Engineering R16-SyllabusSrikanthNo ratings yet
- MIT Mechatronics CurriculumDocument3 pagesMIT Mechatronics CurriculumkausiksharmaNo ratings yet
- For 2008 2009 Batch I SemesterDocument4 pagesFor 2008 2009 Batch I SemesterAneesh ChhabraNo ratings yet
- Result CardDocument1 pageResult CardMuhammad TayyabNo ratings yet
- R-16 - B Tech Petroleum Engineering SyllabusDocument163 pagesR-16 - B Tech Petroleum Engineering SyllabussrinuNo ratings yet
- Updated BTech Curriculum-2Document6 pagesUpdated BTech Curriculum-2sansNo ratings yet
- SCHEME OF COURSES 2nd Year PDFDocument261 pagesSCHEME OF COURSES 2nd Year PDFAditya SharmaNo ratings yet
- Curriculum Syllabus Btech Chemical Engg 2015 Regulations PDFDocument123 pagesCurriculum Syllabus Btech Chemical Engg 2015 Regulations PDFSiva VigneswaranNo ratings yet
- DD Curriculum - TFEDocument5 pagesDD Curriculum - TFESaurabh BhaleraoNo ratings yet
- M.Tech in Chemical Engineering (Specialization: Petroleum Science and Technology)Document43 pagesM.Tech in Chemical Engineering (Specialization: Petroleum Science and Technology)Mriganabh SarmaNo ratings yet
- B.tech Chem 6-8 Aff26042010Document33 pagesB.tech Chem 6-8 Aff26042010Adithya KrishnanNo ratings yet
- Curriculum of B. Tech (Chemical Engineering)Document35 pagesCurriculum of B. Tech (Chemical Engineering)Durga Prasad MoharanaNo ratings yet
- Scheme and Syllabus: - I (CH3XX) - I (CH3AA)Document53 pagesScheme and Syllabus: - I (CH3XX) - I (CH3AA)Abhi PNo ratings yet
- Course Structure DD Latest IITBDocument34 pagesCourse Structure DD Latest IITBBrindaban SahaNo ratings yet
- IITB Chemical Engineering CarriculumDocument20 pagesIITB Chemical Engineering CarriculumpuneetNo ratings yet
- Chemical EngineeringDocument120 pagesChemical EngineeringPhani ChinnaNo ratings yet
- Mech Syllabus 207 Credits PDFDocument185 pagesMech Syllabus 207 Credits PDFAdiNo ratings yet
- Course Curriculum For The New Programme (Dual Degree Tfe, Cada and Cim) W.E.F. 2007 BatchDocument26 pagesCourse Curriculum For The New Programme (Dual Degree Tfe, Cada and Cim) W.E.F. 2007 BatchMainak SahaNo ratings yet
- Mechanical Engineering - R2015 - Ug FT - Curriculum and SyllabusDocument152 pagesMechanical Engineering - R2015 - Ug FT - Curriculum and SyllabusPrabhaharMuthuswamyNo ratings yet
- Curriculam..Ce NitjDocument76 pagesCurriculam..Ce NitjRAMAVATH BABUNo ratings yet
- Updated DD CurriculummainDocument20 pagesUpdated DD CurriculummainAkash MeenaNo ratings yet
- Mechaniical EngineeringDocument5 pagesMechaniical EngineeringChristina RobinsonNo ratings yet
- TIU Diploma 2nd SEMDocument13 pagesTIU Diploma 2nd SEMTechno India UniversityNo ratings yet
- Btech Mechanical 2nd Year 1624713317Document44 pagesBtech Mechanical 2nd Year 1624713317Himansu BisoiNo ratings yet
- Compiled Sylla - Petro II Year 1Document34 pagesCompiled Sylla - Petro II Year 1barhatekrudhnaNo ratings yet
- Chhattisgarh Swami Vivekanand Technical University, Bhilai Scheme of Teaching & ExaminationDocument15 pagesChhattisgarh Swami Vivekanand Technical University, Bhilai Scheme of Teaching & ExaminationSiddharth VermaNo ratings yet
- Automobile Engineering PDFDocument151 pagesAutomobile Engineering PDFrahul chowdaryNo ratings yet
- 7 TE Chemical Syllabus15 - FinalDocument35 pages7 TE Chemical Syllabus15 - FinalSanjeev PawarNo ratings yet
- Department of Chemical Engineering Proposed New Course Structure (B. Tech. in Chemical Engineering)Document33 pagesDepartment of Chemical Engineering Proposed New Course Structure (B. Tech. in Chemical Engineering)Akash BodekarNo ratings yet
- Academic Regulations Course Structure and Detailed Syllabus: (Two Years Full Time Programme)Document55 pagesAcademic Regulations Course Structure and Detailed Syllabus: (Two Years Full Time Programme)Vincent LinzieNo ratings yet
- Industrialrefrcryo-03 11 15Document72 pagesIndustrialrefrcryo-03 11 15ASHITA K BNo ratings yet
- Bachelor of Technology: (Course Structure and Syllabi) ForDocument53 pagesBachelor of Technology: (Course Structure and Syllabi) ForFraud StaanNo ratings yet
- REGULATIONS - 2015 (R2015) (CBCS) : Kumaraguru College of Technology Coimbatore - 641 049Document166 pagesREGULATIONS - 2015 (R2015) (CBCS) : Kumaraguru College of Technology Coimbatore - 641 049Anonymous pRytMnCrDNo ratings yet
- Subjects Credit Hrs Credit Hours Theory Lab: BSME Scheme of StudiesDocument6 pagesSubjects Credit Hrs Credit Hours Theory Lab: BSME Scheme of StudiesAmir Khan YousafzaiNo ratings yet
- Srmap Mech 2020 2024 Syllabus Non UgcDocument159 pagesSrmap Mech 2020 2024 Syllabus Non UgcKick ButtowskiNo ratings yet
- CBSC Syllabus Mech II Yr26!08!2017Document53 pagesCBSC Syllabus Mech II Yr26!08!2017Milan MottaNo ratings yet
- Me 1&2yearscs & Syllabus Ug r20Document71 pagesMe 1&2yearscs & Syllabus Ug r20LuckyNo ratings yet
- Guidelines for Chemical Process Quantitative Risk AnalysisFrom EverandGuidelines for Chemical Process Quantitative Risk AnalysisRating: 5 out of 5 stars5/5 (1)
- Process Analytical Technology: Spectroscopic Tools and Implementation Strategies for the Chemical and Pharmaceutical IndustriesFrom EverandProcess Analytical Technology: Spectroscopic Tools and Implementation Strategies for the Chemical and Pharmaceutical IndustriesKatherine A. BakeevNo ratings yet
- Plumbing and Piping Systems Inspection Notes: Up to CodeFrom EverandPlumbing and Piping Systems Inspection Notes: Up to CodeRating: 3 out of 5 stars3/5 (2)
- End Sem Practical May2024Document2 pagesEnd Sem Practical May2024Akhil KumarNo ratings yet
- LLM For QnA ProposalDocument12 pagesLLM For QnA ProposalAkhil KumarNo ratings yet
- Prsentation Schedule (CH-327)Document2 pagesPrsentation Schedule (CH-327)Akhil KumarNo ratings yet
- Annex I-ADocument1 pageAnnex I-AAkhil KumarNo ratings yet
- Annex IIDocument10 pagesAnnex IIAkhil KumarNo ratings yet
- Akhil SeminarDocument14 pagesAkhil SeminarAkhil KumarNo ratings yet
- B. Tech. Seminar Report: Recycling of PlasticsDocument31 pagesB. Tech. Seminar Report: Recycling of PlasticsAkhil KumarNo ratings yet
- MT 2Document18 pagesMT 2Akhil KumarNo ratings yet
- Pi7 Tool PDFDocument1 pagePi7 Tool PDFAkhil KumarNo ratings yet
- 111145Document1 page111145Akhil KumarNo ratings yet
- StackDocument48 pagesStackAkhil KumarNo ratings yet
- Dsa NotesDocument109 pagesDsa NotesAkhil KumarNo ratings yet
- Non-Ideal Flow: Residence Time DistributionDocument71 pagesNon-Ideal Flow: Residence Time DistributionAkhil KumarNo ratings yet
- Introduction To Plastic RecyclingDocument10 pagesIntroduction To Plastic RecyclingAkhil KumarNo ratings yet
- PDC Es5Document18 pagesPDC Es5Akhil KumarNo ratings yet
- PDC Es3Document25 pagesPDC Es3Akhil KumarNo ratings yet
- PDC Es1Document25 pagesPDC Es1Akhil KumarNo ratings yet
- Linked ListDocument87 pagesLinked ListAkhil KumarNo ratings yet
- AkhilDocument10 pagesAkhilAkhil KumarNo ratings yet
- Linked SQDocument43 pagesLinked SQAkhil KumarNo ratings yet
- KVB Application Form (A, B, C, D) ÅB-TëçhzDocument6 pagesKVB Application Form (A, B, C, D) ÅB-TëçhzAkhil KumarNo ratings yet
- Chemical TechnolgyDocument39 pagesChemical TechnolgyAkhil KumarNo ratings yet
- MalachiteDocument2 pagesMalachiteAkhil KumarNo ratings yet
- Chapter 1 - Measurements in ChemistryDocument4 pagesChapter 1 - Measurements in ChemistryChristelle Ng Li Xuan (Student)No ratings yet
- Outdoor-Eic Understanding Heatload EbookDocument9 pagesOutdoor-Eic Understanding Heatload EbookPeggy PattenNo ratings yet
- Hvac Precoling Eng WebDocument2 pagesHvac Precoling Eng WebShaaban HassanNo ratings yet
- Sheet Ch.1Document4 pagesSheet Ch.1Ahmed KingNo ratings yet
- IB HL Chemistry Assessment Statements Topic 5Document4 pagesIB HL Chemistry Assessment Statements Topic 5AndrewNo ratings yet
- Experiment 21: Joule's Law: PurposeDocument4 pagesExperiment 21: Joule's Law: PurposelodhiNo ratings yet
- lm196 396Document14 pageslm196 396Raedwulf0No ratings yet
- ASTM D1525-09 Standard Test Method For Vicat Softening Temperature of PlasticsDocument10 pagesASTM D1525-09 Standard Test Method For Vicat Softening Temperature of PlasticsalexintelNo ratings yet
- P&ID DRAWING 1 New (Pepsi-Chiller System)Document1 pageP&ID DRAWING 1 New (Pepsi-Chiller System)Hồng Quang Lê100% (1)
- Honikel 1998 Reference Methods For The Assessment of Physical in MeatDocument11 pagesHonikel 1998 Reference Methods For The Assessment of Physical in MeatFood Science & Technology100% (3)
- FluidandThermodynamics Vol2Document21 pagesFluidandThermodynamics Vol2GodNo ratings yet
- Touch-N-Heat Ifu 300-552 Rev-F Trim WebDocument39 pagesTouch-N-Heat Ifu 300-552 Rev-F Trim WebShawn CarswellNo ratings yet
- Energy Entropy and The Flow of Nature Thomas F Sherman Full ChapterDocument67 pagesEnergy Entropy and The Flow of Nature Thomas F Sherman Full Chapterdenise.sweeney729100% (10)
- LWT - Food Science and TechnologyDocument7 pagesLWT - Food Science and TechnologyHarold RosalesNo ratings yet
- 1305-V1211 R410A DC Inverter VRF SERIES 60HzV4+ - V4+S - V4+R - V4+W - Mini VRF) PDFDocument63 pages1305-V1211 R410A DC Inverter VRF SERIES 60HzV4+ - V4+S - V4+R - V4+W - Mini VRF) PDFStefy CarrascoNo ratings yet
- Performance and Improvement of Cleanroom Environment Control System Related To Cold-Heat Offset in Clean Semiconductor FabsDocument13 pagesPerformance and Improvement of Cleanroom Environment Control System Related To Cold-Heat Offset in Clean Semiconductor Fabsqac gmpNo ratings yet
- DVS 2207-12 Soldadura de Termoplasticos - Soldadura de Tubos y Accesorios de PVC-UDocument7 pagesDVS 2207-12 Soldadura de Termoplasticos - Soldadura de Tubos y Accesorios de PVC-UEugenioNo ratings yet
- Forming Process and Numerical Simulation of Making Upset On Oil Drill PipeDocument9 pagesForming Process and Numerical Simulation of Making Upset On Oil Drill PipeBepdjNo ratings yet
- ASTM D1525 - Standard Test Method For Vicat Softening Temperature of Plastics PDFDocument9 pagesASTM D1525 - Standard Test Method For Vicat Softening Temperature of Plastics PDFArnelNo ratings yet
- Catalogue MEGA HISSOTTO MAROCDocument37 pagesCatalogue MEGA HISSOTTO MAROCKatcherr CherrNo ratings yet
- KFCL Summer ProjectDocument59 pagesKFCL Summer ProjectSumitMohanGuptaNo ratings yet
- ABB Review NR 3 2013Document84 pagesABB Review NR 3 2013sorin1gunnerNo ratings yet
- Lennox Cbx27uh Air Handler DataDocument28 pagesLennox Cbx27uh Air Handler DataAENo ratings yet
- Class 7 Heat and EffectsDocument5 pagesClass 7 Heat and Effectsnitin khandelwalNo ratings yet
- GDC 9 RwaDocument9 pagesGDC 9 RwaPete PompesNo ratings yet
- DLL - Science 10 - Q4Document32 pagesDLL - Science 10 - Q4Nazer M. LacaboNo ratings yet
- BS-3 Unit 1Document116 pagesBS-3 Unit 1Pavithra SivasankaranNo ratings yet
- Transport Phenomena: Notes For The 2nd Revised Edition of by R. B. Bird, W. E. Stewart, and E. N. LightfootDocument168 pagesTransport Phenomena: Notes For The 2nd Revised Edition of by R. B. Bird, W. E. Stewart, and E. N. Lightfoot김수한No ratings yet
- 30kav 0451 - 123RTDocument3 pages30kav 0451 - 123RTPattamon KhempilaNo ratings yet