Review
Abstract
Background: An understanding of the technology acceptance of home-based cardiac telerehabilitation programs is paramount if they are to be designed and delivered to target the needs and preferences of patients with coronary heart disease; however, the current state of technology acceptance of home-based cardiac telerehabilitation has not been systematically evaluated in the literature.
Objective: We aimed to provide a comprehensive summary of home-based cardiac telerehabilitation technology acceptance in terms of (1) the timing and approaches used and (2) patients’ perspectives on its usability, utility, acceptability, acceptance, and external variables.
Methods: We searched PubMed, CENTRAL, Embase, CINAHL, PsycINFO, and Scopus (inception to July 2021) for English-language papers that reported empirical evidence on the technology acceptance of early-phase home-based cardiac telerehabilitation in patients with coronary heart disease. Content analysis was undertaken.
Results: The search identified 1798 studies, of which 18 studies, with 14 unique home-based cardiac telerehabilitation programs, met eligibility criteria. Technology acceptance (of the home-based cardiac telerehabilitation programs) was mostly evaluated at intra- and posttrial stages using questionnaires (n=10) and usage data (n=11). The least used approach was evaluation through qualitative interviews (n=3). Usability, utility, acceptability, and acceptance were generally favored. External variables that influenced home-based cardiac telerehabilitation usage included component quality, system quality, facilitating conditions, and intrinsic factors.
Conclusions: Home-based cardiac telerehabilitation usability, utility, acceptability, and acceptance were high; yet, a number of external variables influenced acceptance. Findings and recommendations from this review can provide guidance for developing and evaluating patient-centered home-based cardiac telerehabilitation programs to stakeholders and clinicians.
doi:10.2196/34657
Keywords
Introduction
Within the spectrum of cardiovascular diseases, coronary heart disease is the most common cause of mortality and morbidity globally and presents a major health care burden []. Cardiac rehabilitation is a widely accepted treatment modality for secondary prevention of coronary heart disease [], but long-standing challenges regarding accessibility to cardiac rehabilitation facilities, conflicting work and care responsibilities, low socioeconomic status, and costs of rehabilitation programs have led to disappointingly low reported uptake rates among eligible patients worldwide (10% to 30% []). A recent challenge is the COVID-19 pandemic []. In the acute phase of the pandemic, nonurgent outpatient services, such as center-based cardiac rehabilitation, were partially or completely closed as limited resources and personnel were redirected to critical areas. Even in the long-term phase of the pandemic, efforts to limit the spread of COVID-19 infection through measures such as safe distancing further limited the capacity for delivery of center-based cardiac rehabilitation group exercise and therapy sessions []. Thus, alternative secondary prevention strategies for coronary heart disease are a priority across health care systems during the COVID-19 pandemic and beyond [].
Home-based cardiac telerehabilitation—defined as the use of information and communication technologies (eg, mobile- and web-based platforms, wearable sensor devices) to deliver remote exercise supervision, education, counseling on cardiovascular risk factor modification, and psychosocial support exclusively at home—is one such emerging alternative []. A recent systematic review and meta-analysis [] of randomized controlled trials comparing home-based cardiac telerehabilitation to center-based cardiac rehabilitation in patients with coronary heart disease found equivalent effects on functional capacity, cardiac-related hospitalization, physiological risk factor control, quality of life, depression, and behaviors such as physical activity, smoking cessation, and medication adherence. However, adapting digital solutions for health problems is not without its challenges; attempts to scale up effective digital health research interventions into real-world health care systems have been met with difficulty, especially for complex interventions that require user interaction [,]. The successful incorporation of such digital health technologies into clinical practice is contingent upon end-users’ (ie, patients) acceptance and sustained engagement with the intervention, and thus, these are important aspect for researchers, health care systems, and policymakers to consider [,].
The technology acceptance model provides a framework for modeling end-user acceptance [] and theorizes that both perceived usefulness (ie, utility) and perceived ease of use (ie, usability) of a target system directly influence intention to use (ie, acceptability), which then influences actual system use (ie, acceptance of the system) []. External variables such as technology self-efficacy and training, objective system design features, and the process of system implementation are thought to indirectly influence system acceptability and acceptance by influencing system utility and usability []. An understanding of the usability and utility of home-based cardiac telerehabilitation programs is paramount if they are to be designed and delivered to target the needs of patients with coronary heart disease in a way that ensures programs are accepted. However, the current state of technology acceptance of home-based cardiac telerehabilitation has not been systematically evaluated in the literature. We aimed to provide a comprehensive summary of the technology acceptance of home-based cardiac telerehabilitation among patients with coronary heart disease.
Methods
Study Design
We performed a systematic scoping review to comprehensively collate, summarize, and map [,] existing evidence on home-based cardiac telerehabilitation research in terms of usability, utility, acceptability, and acceptance testing. We used the Arksey and O’Malley methodological framework []: identifying the research questions, identifying relevant studies, study selection, charting the data, collating, summarizing, and reporting the results. To ensure quality and transparency, this review was conducted and reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses Scoping Review guidelines []. A review protocol (not registered) was prepared prior to the start of this review.
Identifying the Research Question
The following research questions were identified to answer the objective of this review: (1) What are the timing and approaches used to evaluate the technology acceptance attributes in home-based cardiac telerehabilitation? (2) What are patients’ perspectives on the technology acceptance constructs (ie, usability, utility, acceptability, acceptance, and external variables) of home-based cardiac telerehabilitation?
Identifying Relevant Studies
We followed recommendations by Arksey and O‘Malley [] and undertook an iterative approach, through ongoing consultations with a university resource librarian throughout the search process, to identify relevant literature. We piloted an initial search strategy in PubMed and EMBASE to identify a sample of relevant papers. This was followed by an analysis of the keywords used in the titles and abstracts and in the indexing of these relevant papers. Preliminary results revealed that terms related to the concept acceptance were not commonly indexed in relevant papers. Thereafter, we used terms related to coronary heart disease, rehabilitation, and telehealth. We searched PubMed, Cochrane Central Register of Controlled Trials, Embase, Cumulative Index to Nursing and Allied Health Literature, PsycINFO, and Scopus databases (inception to July 2021). No limits on study design were placed. Additionally, we manually searched the reference lists of relevant systematic reviews and papers included in this review (Table S1 in ).
Study Selection
Overview
Literature evaluating the technology acceptance constructs of home-based cardiac telerehabilitation that used empirical methods (both quantitative and qualitative) and were published in English were considered. Case reports, conference abstracts, editorials, protocols, and reviews were excluded. The PCC (Population, Concept, Context) framework [] was used to develop and set the inclusion and exclusion criteria. Search results were imported to Endnote (version X9, Clarivate Analytics) for management. Two independent authors were involved in the study selection process. Records deemed relevant by both authors were included. Consultation with a third author was used to resolve any disagreements regarding inclusion.
Population
Papers with a study population of patients with a documented medical diagnosis of coronary heart disease, acute coronary syndrome, myocardial infarction, angina pectoris, or who had undergone revascularization (ie, coronary artery bypass grafting or percutaneous coronary intervention) were included. We excluded papers with a study population of patients with heart failure (regardless of left ventricular ejection fraction), as their therapeutic needs and subsequent evaluations of home-based cardiac telerehabilitation in terms of usability, utility, acceptability, acceptance, and external variables would differ considerably from those of patients with coronary heart disease.
Concept
For the purpose of this study, the constructs of the technology acceptance model were conceptualized as follows: (1) usability—degree to which the system is easy to use and free of effort; (2) utility—degree to which the system improves user’s performance and functions as intended; (3) acceptability—behavioral intention or willingness to use the system; and (4) acceptance—actual usage of the system [,]. Home-based cardiac telerehabilitation was defined as any mobile health app or website used either as a stand-alone platform or supplemented with other modes of delivery, such as telephone or video calls, short message service, email, or telemonitoring, to exclusively deliver early cardiac rehabilitation or secondary prevention []. The decision to focus on mobile- or web-based home-based cardiac telerehabilitation was made with the purpose of scoping the technologies that allowed for greater interaction, flexibility, and independence in rehabilitation programs. Papers were included if they addressed the testing and evaluation of technology acceptance constructs from patient perspectives. Late-phase home-based cardiac telerehabilitation programs, in which the focus is placed on long-term maintenance of lifestyle change, were excluded since we were only interested in the early and active rehabilitation phase (ie, focus on health behavior change, risk factor modification and psychosocial well-being.).
Context
The context for telerehabilitation programs was limited to those in a home setting only; hence, we excluded home-based cardiac telerehabilitation delivered alongside center-based cardiac rehabilitation (ie, hybrid cardiac rehabilitation services).
Charting the Data
Authors, publication year, country of origin, study design, subcategory of coronary heart disease population, sample size, characteristics of home-based cardiac telerehabilitation program, approach, and timing of technology acceptance evaluation data were extracted by the first author and confirmed by the second author, who made adjustments and included additional information where necessary. Features of the home-based cardiac telerehabilitation programs were categorized according to recommendations by Whitelaw et al [] to facilitate uptake of digital health interventions. We categorized the core components present in the home-based cardiac telerehabilitation programs using American Heart Association classifications [].
Collating, Summarizing, and Reporting the Findings
We used a 3-phase process [] to systematically conduct our content analysis of the technology acceptance of home-based cardiac telerehabilitation among patients with coronary heart disease: preparing, organizing, and reporting of data. Because the aim of the review was to structure a descriptive analysis of home-based cardiac telerehabilitation acceptance based on the constructs of the technology acceptance model, we used a deductive content analysis approach [,].
A structured categorization matrix was prepared based on the constructs of the technology acceptance model: usability, utility, acceptability, acceptance, and external variables. Two authors concurrently and independently reviewed all the studies for content and coded data that corresponded to categories in the matrix. Content that did not fit into the other categories was gathered and coded under the category external variables and was analyzed based on the principle of inductive content analysis—open coding was undertaken, and data were grouped into similar categories and labeled with subcategories using content-characteristic words []. After data were organized, each author reviewed all of the studies under each category to check the reliability of the content analysis process and identify discrepancies in the collating and categorization of study data. Discussions were held until both authors were in agreement with the content under each category. Consultation with a third author was used to resolve any disagreements. The timing of home-based cardiac telerehabilitation evaluations was categorized based on when evaluation was undertaken relative to the trial implementation stage—pretrial, intratrial, or posttrial.
Quality appraisal was not performed as the objective of this scoping review was to provide an overview of the existing evidence on the evaluations of usability, utility, acceptability, and acceptance in home-based cardiac telerehabilitation, regardless of the quality of the evidence [].
Results
General
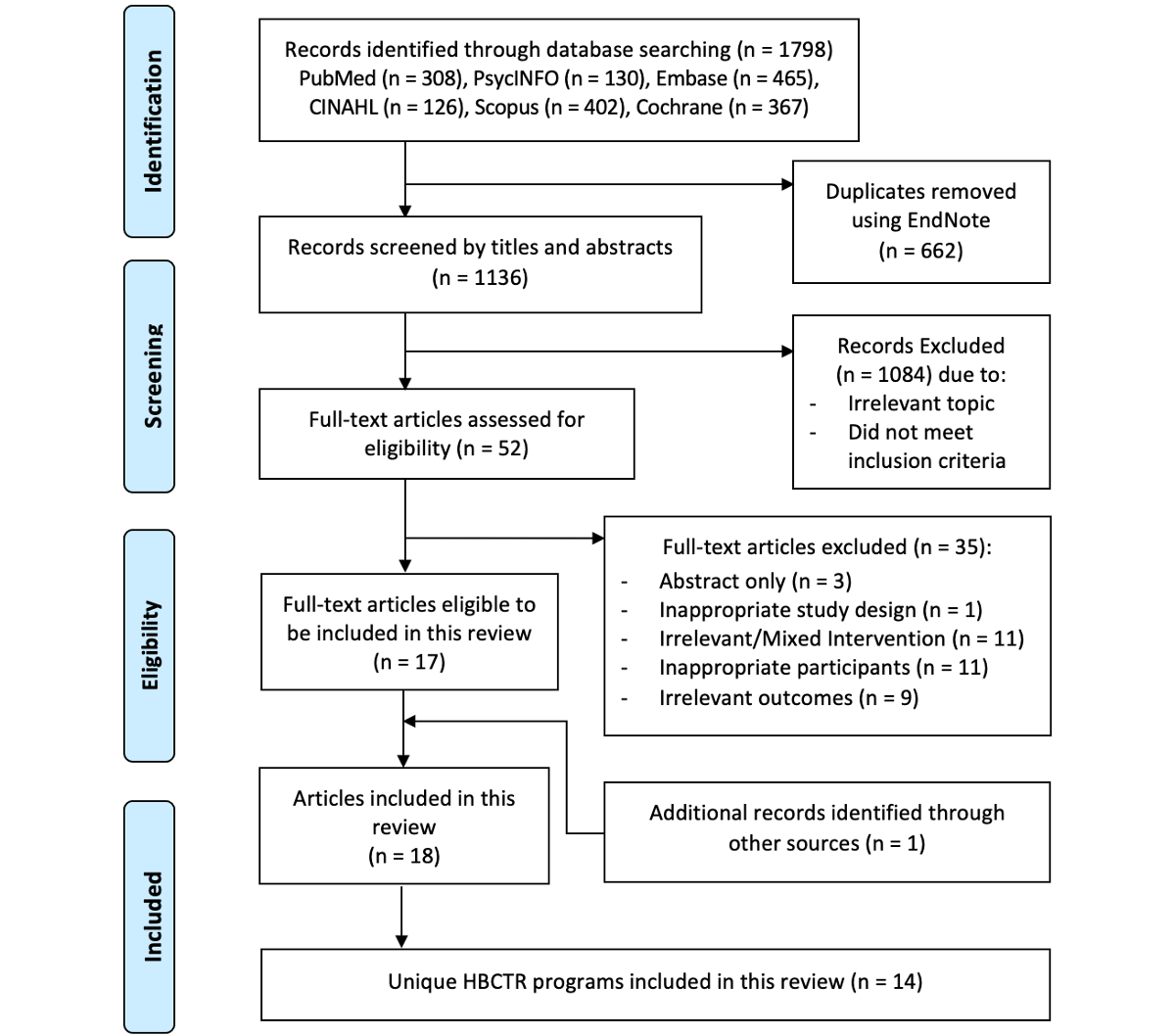
The search generated 1136 unique papers. After title and abstract screening, 1084 papers were excluded. The remaining 52 full-text papers were retrieved and screened, and 35 papers were excluded (Table S2 in ). Manual searches of the reference lists of relevant papers identified 1 paper for inclusion; therefore, 18 papers [-], with 14 independent home-based cardiac telerehabilitation programs, were included in this review ().
Characteristics of Studies
Studies included in this review (Table S3 in ) were published between 2007 and 2021; the majority (n=14) were published after 2013. Studies were conducted in the following countries: China [,,,,,]; Australia [,,,]; Canada [-]; United States of America [,]; United Kingdom [,]; and New Zealand []. Studies included patients who had the following: stable angina; myocardial infarction; stable coronary heart disease; or underwent coronary revascularization (ie, coronary artery bypass grafting or percutaneous coronary intervention). In studies that reported age and gender of participants, the mean age of patients ranged from 53 to 66 years and the proportion of female patients ranged from 9.4% to 33%. Devi et al [] and Varnfield et al [] were earlier papers reporting on the same home-based cardiac telerehabilitation programs as those in Devi et al [] and Varnfield et al [], respectively. Zutz et al [] and Lear et al [] were both earlier papers reporting on the same home-based cardiac telerehabilitation program as that in Banner et al [].
Characteristics of Home-Based Cardiac Telerehabilitation Programs
Home-based cardiac telerehabilitation programs were delivered mainly via smartphone apps (n=11) and websites (n=3) and were supplemented by other modes of delivery: text messaging (n=6), telephone calls (n=5), emails (n=2), videoconferencing (n=1), and telemonitoring (n=10). Telemonitoring devices that supported remote supervision of exercise training by the cardiac rehabilitation team and patients’ self-monitoring of physical activity included heart rate monitors, accelerometers, and pedometers.
Features of the home-based cardiac telerehabilitation programs included engagement of stakeholders, clinicians, and patients throughout the design or development of the home-based cardiac telerehabilitation program (n=3); testing of the home-based cardiac telerehabilitation program by cardiology experts and patients (n=8); provision of face-to-face training on use of home-based cardiac telerehabilitation for patients (n=10); ongoing technical support throughout home-based cardiac telerehabilitation program (n=4); and consideration of data privacy and security in the use of technologies in home-based cardiac telerehabilitation (n=7).
The American Heart Association core components [] that were present in the home-based cardiac telerehabilitation programs were patient assessment (n=14), exercise training (n=13), dietary management (n=10), risk factor management (n=11), medication adherence (n=8), and psychosocial support (n=6). Only 5 studies [,,,,] had a comprehensive home-based cardiac telerehabilitation program that included all the core components (Table S4 in ).
Timing and Approaches to Evaluation
Home-based cardiac telerehabilitation programs were commonly evaluated at the pretrial stage (n=5) and using a combination of intra and posttrial measures (n=4), followed by intratrial only (n=3), posttrial only (n=1) and a combination of pre-, intra-, and posttrial measures (n=1) (). The following methods were used to evaluate home-based cardiac telerehabilitation: questionnaires (n=10); usage data (n=11); and interviews (n=3). Except for one study [] that used the System Usability Scale, the remaining questionnaires used were ad hoc surveys. Yu et al [] used a combination of captured usage data and patient-report questionnaires to evaluate acceptance of home-based cardiac telerehabilitation at both the intra- and posttrial stage. Higgins et al [] used both questionnaires and interviews to evaluate both usability and utility of their home-based cardiac telerehabilitation program.

Usability
Of the 18 studies, 7 studies [,-,,,] reported the usability of home-based cardiac telerehabilitation programs. Specific outcomes measures within the usability construct included perceived ease of system use and navigation [,,,], ease and comfort of use of wearable devices [], system learnability [,], and comprehension and ease of undertaking tasks on the system [,]. Overall, studies reported high usability rating scores and qualitative feedback from participants regarding home-based cardiac telerehabilitation use.
Utility
Specific outcomes measures within the utility construct included perceived usefulness in supporting behavior change [,,,,], in managing psychological well-being [,], in controlling symptoms [], in tracking goals and progress [,-,], in reducing outpatient visits [], and of the overall home-based cardiac telerehabilitation system [,,]. Utility of home-based cardiac telerehabilitation was generally favorably perceived, with the exception of 2 studies [,] in which perceived usefulness of the system was rated poorly.
Acceptability
High rates of acceptability were reported in 3 studies [,,], ranging from 81.3% to 88% of participants who agreed that they would continue to use the home-based cardiac telerehabilitation system regularly after they had completed the study intervention period. Prior to system use, one study [] reported an acceptability rate of 59.3% (participants who were potentially willing to participate in a home-based cardiac telerehabilitation program).
Acceptance
Most studies reported participants’ usage of the home-based cardiac telerehabilitation system either through direct evaluation of program usage data or through self-reported participant survey responses (). Studies included a very broad range of outcome measures including engagement with home-based cardiac telerehabilitation [,,,,,] (ie, frequency and volume of website log-ins, smartphone app usage, activity tracker wear time); tasks completed [,,,,] (ie, frequency and volume of educational modules reviewed, vitals logged, counseling sessions attended, response to program reminders), and captured exercise data [,,,,,] (ie, objective telemonitoring data on the uptake, adherence, and completion of prescribed exercise sessions and goals). Overall, usage was high, reflecting high end-user acceptance. Only 5 studies [,,,,] reported usage data for specific components over time to determine the timepoints when participant usage tapered or ceased ().
| Method, definition of actual use, and data timepoint | Acceptance of home-based cardiac telerehabilitation program | |||
| Program usage data | ||||
| Engagement with home-based cardiac telerehabilitation program | ||||
| 6 weeks |
| |||
| 12 weeks |
| |||
| 16 weeks |
| |||
| 24 weeks |
| |||
| Tasks completed | ||||
| 12 weeks |
| |||
| 16 weeks |
| |||
| 24 weeks |
| |||
| 52 weeks |
| |||
| Captured exercise data | ||||
| 6 weeks |
| |||
| 8 weeks |
| |||
| 12 weeks |
| |||
| 24 weeks |
| |||
| Self-reported survey responses | ||||
| Engagement with home-based cardiac telerehabilitation program | ||||
| 24 weeks |
| |||
| Tasks completed | ||||
| 24 weeks |
| |||
aUptake was defined as attending baseline assessment, and uploading exercise data once to the home-based cardiac telerehabilitation platform.
bAdherence was defined as uploading 4 weeks of exercise data onto the home-based cardiac telerehabilitation.
cCompletion was defined as attendance at the 6-week assessment.
dCompletion was defined as attendance at the 8-week assessment.
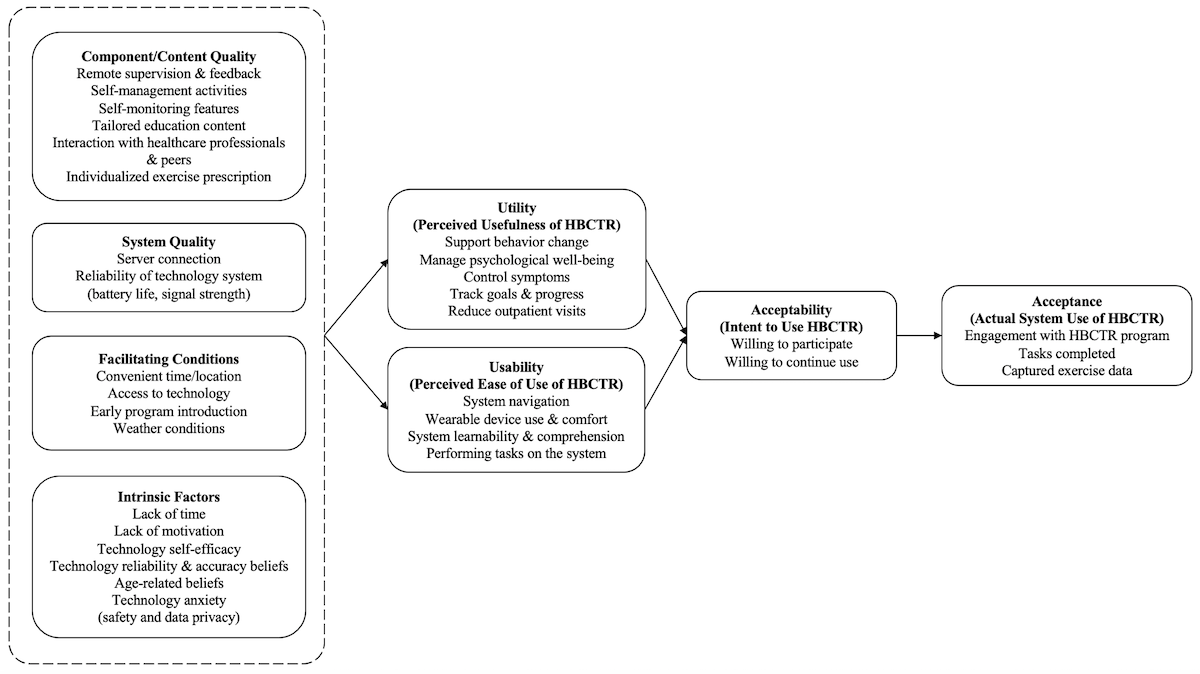
External Variables
Component Quality
The majority of the existing literature (n=8) on home-based cardiac telerehabilitation evaluation reported the program components that participants valued: remote supervision and feedback [,,-,], support for self-management and self-monitoring [,,,,], range of relevant educational modules [,,], ability to communicate with health care professionals [,,], and individualized exercise prescription []. Participants desired more interactive components such as chat platforms and noticeboards with peers to facilitate peer interaction and support [,,] and greater intra- and postprogram support []. Participants in one study [] wanted specific education content pertaining to death anxiety, and content that aligned rehabilitation goals with the purpose of living.
System Quality
Two studies [,] detailed participants’ perspectives on the technical efficiency of the home-based cardiac telerehabilitation system. Specifically, issues relating to server connection and reliability of the technology (ie, equipment battery life and signal strength) were reported as these influenced participants ability to engage with the program without interruption.
Intrinsic Factors
Participants reported several intrinsic factors at the individual level that influenced how they perceived home-based cardiac telerehabilitation programs (n=5). These included lack of time [,,], lack of motivation [,,], perceived self-efficacy in operating the telerehabilitation system [], perceived reliability and accuracy of technology [], apprehension related to safety and data privacy [], and preconceived beliefs regarding the suitability of home-based cardiac telerehabilitation for older age [,].
Facilitating Conditions
The existence of resource and situational factors facilitated the usage of home-based cardiac telerehabilitation programs in included studies (n=5). Participants valued the accessibility and convenience offered by home-based cardiac telerehabilitation as it overcame restrictions related to time and location [,,], but some expressed that regular access to the internet and computers would have facilitated uninterrupted usage of the program in earlier studies [,]. Situational factors such as timing of program introduction also influenced participants’ perception of home-based cardiac telerehabilitation usage [,]. Participants reported wanting the program to begin sooner after their diagnosis to facilitate early establishing of routines and prevent potential cardiac complications [,]. Wet and cold seasons were reported as a barrier to outdoor physical exercises [].
Details of the external variables, usability, utility, and acceptability of home-based cardiac telerehabilitation reported in included studies can be found in Table S5 ().
Discussion
Principal Findings
In our scoping review, we found that most evaluations were undertaken at the intratrial and posttrial stage using singular methodological approaches, and although home-based cardiac telerehabilitation had high usability, utility, acceptability, and acceptance, patients reported a number of external variables such as component quality, system quality, intrinsic factors, and facilitating conditions that influenced how they interacted with the home-based cardiac telerehabilitation program.
Timing of Home-Based Cardiac Telerehabilitation Evaluation
Early evaluation of end-user acceptance and feasibility issues can critically inform the development and design of digital interventions and mitigate risks that an intervention is later undesirable or even abandoned at trial implementation stages [,]. Through our scoping review, we found that the majority of home-based cardiac telerehabilitation programs reported evaluations of technology acceptance either during or after trial implementation; evaluations were rarely reported at the pretrial stage. This may reflect a tendency in implementation research to prioritize the evaluation of trial intervention effectiveness over trial implementation effectiveness []. Yet, achieving intended trial effects is greatly dependent on participants’ sufficient engagement with the implemented technology in a trial that strongly appeals to their contextual health care needs []. Hence, there is a need for future research on home-based cardiac telerehabilitation to refocus efforts of program evaluation more upstream, so that identified technology acceptance issues can be addressed and programs finetuned to ensure optimal success before trial implementation.
Approaches to Home-Based Cardiac Telerehabilitation Evaluation
Our review of the methodological approaches used to evaluate the technology acceptance constructs in home-based cardiac telerehabilitation revealed 3 main concerns. First, although home-based cardiac telerehabilitation programs used either quantitative (ie, survey questionnaires) or qualitative (ie, interviews) approaches to evaluate usability, utility, and acceptability, only 3 studies [,,] employed qualitative methods (), and only one study [] used both approaches in tandem to evaluate the same technology acceptance attribute. Questionnaires are usually inexpensive and useful in gathering quantitative data in large samples but lack the ability to facilitate comprehension of in-depth individual variation in behaviors, perspectives, and experiences that qualitative interviews provide []. Such information is crucial to designing and delivering home-based cardiac telerehabilitation programs that truly match patients’ needs and preferences. We recommend that future home-based cardiac telerehabilitation programs employ a mixed methods approach, comprising both quantitative and qualitative methods to guarantee evaluation results that are practical, interpretable, and comprehensive [].
Second, apart from one study [] that used the System Usability Scale questionnaire, the remaining home-based cardiac telerehabilitation programs in this review used customized ad hoc questionnaires to measure the constructs of technology acceptance. This corresponds with the findings of previous reviews [,], which mostly included studies that evaluated digital health technology acceptance attributes using quantitative measures that lacked the psychometric properties of reliability and validity. This finding highlights an apparent scarcity of validated tools to evaluate technology acceptance in the context of digital health []. Furthermore, this could reflect the need for researchers to develop their own questionnaires that consider program-specific components, with general acceptance concepts, to allow for an assessment of technology acceptance attributes that is tailored to the particular home-based cardiac telerehabilitation context and population. However, this makes comparing results across studies challenging. It would be commendable to see future research efforts dedicated to adapting existing questionnaires or even validating new tools that encompass the unique home-based cardiac telerehabilitation context. We believe that having such generalizable measures can greatly advance home-based cardiac telerehabilitation research and practice by creating opportunities for comparable data on technology acceptance constructs to be analyzed and for comparative benchmarks to be set in home-based cardiac telerehabilitation program evaluation.
Third, home-based cardiac telerehabilitation programs had varied definitions and measurements of acceptance (ie, actual system usage) (). This is consistent with previous literature on the use of digital health technologies for cardiovascular disease self-management [] and may be indicative of attempts to examine the multifarious behavior changes addressed in cardiac rehabilitation. Given that user engagement with technology is a dynamic process occurring in a self-directed manner by which users continually decide to either use or abandon a technology system [,], evaluations of home-based cardiac telerehabilitation acceptance should account for this temporal nature and analyze how usage evolves over the course of the rehabilitation program. This is especially important as interventions such as home-based cardiac telerehabilitation are theorized to require sustained use over time to realize intended effects. However, only 5 home-based cardiac telerehabilitation programs [,,,,] reported usage over time (date-tagged acceptance data). Gallagher and Zhang [] recommend the clear identification of individual digital health components targeted at behavior change and the integration of software capabilities that can monitor the usage of respective components. As the eventual goal of home-based cardiac telerehabilitation programs is successful incorporation into clinical practice, it would be interesting to see future studies examine the causal relationships between the level of home-based cardiac telerehabilitation usage and objective intervention outcome over time to determine the specific dose of a home-based cardiac telerehabilitation component needed to achieve optimal behavioral, physiological, and clinical outcomes.
Technology Acceptance of Home-Based Cardiac Telerehabilitation
The acceptance rates observed in our review could be explained by the high usability, utility, and acceptability reported in the programs and correspond to the fundamental basis of the technology acceptance model, that is, that technology acceptance is determined by the degree of value and perceived burden []. This finding not only offers validation to the technology acceptance model but points to the potential of home-based cardiac telerehabilitation to revolutionize the landscape of secondary prevention by blending traditional services provided by health care professionals with technology-enabled self-care platforms to continue the provision of patient-centered care. This is especially crucial during the current COVID-19 pandemic to mitigate the demand for in-person services []. The suitability of home-based cardiac telerehabilitation as an effective alternative to center-based cardiac rehabilitation has been recently reported [], with prospects for significant economic cost-savings through improved productivity and health outcomes []. Yet, an evaluation of end-user acceptance is foundational if barriers and gaps to patient uptake are to be addressed, and if successful wide-scale implementation of home-based cardiac telerehabilitation into clinical practice is to be realized. In the context of home-based cardiac telerehabilitation for patients with coronary heart disease, our review underlined the external variables that have influenced patient’s perceived usability and utility of home-based cardiac telerehabilitation. Recommendations for addressing these variables are offered in in the following paragraphs and may serve to provide a foundation for the development and design of future home-based cardiac telerehabilitation programs ().
| Topic | Recommendation |
| Evaluation timing | Home-based cardiac telerehabilitation program evaluation should be undertaken throughout the entirety of the developmental and implementation, ie, before, during and after trial implementation. |
| Evaluation approach | Home-based cardiac telerehabilitation program evaluation should employ a mixed approach comprising of both quantitative and qualitative methods. Measurement tools must be tailored to encompass the unique context of home-based cardiac telerehabilitation by adapting existing questionnaires or validating new ones. Evaluations of home-based cardiac telerehabilitation technology acceptance should analyze how usage of individual program components evolves over the course of the rehabilitation program. Causal relationships between home-based cardiac telerehabilitation usage and intervention outcomes should be examined to determine specific doses needed to achieve optimal behavioral, physiological, and clinical outcomes. |
| Design and testing | Developers should prioritize user-centered approaches by partnering with end users (ie, clinicians and patients) in the co-designing of programs in the early stages of program design. Field-testing and evaluations of the technologies supporting home-based cardiac telerehabilitation services should occur prior to trial implementation stages. |
| Individualization | Home-based cardiac telerehabilitation programs should be offered as early as possible for patients. Alternatives for either indoor or outdoor exercise training should be programmed. |
| Accessibility | Home-based cardiac telerehabilitation programs should be adapted to the socioeconomic needs of end users and their community Partnerships with local governing bodies should be established to marshal resources and secure funding to invest in required infrastructure. The prospects of insurance coverage for home-based cardiac telerehabilitation programs should be explored. home-based cardiac telerehabilitation programs should be reasonably priced with subsidies for mobile phones, data plans and wearables. |
| Data privacy and security | Home-based cardiac telerehabilitation should provide patients with transparent privacy policies and comply with data governance regulations and security protocols. |
| Training | Patients should be provided introductory training sessions that are supported by practical step-by-step instruction manuals. |
| Technology support | Designated technical support staff should be made available on home-based cardiac telerehabilitation platforms. |
Recommendations for Home-Based Cardiac Telerehabilitation Development
Home-based cardiac telerehabilitation developers should prioritize user-centered approaches by partnering with end users (ie, clinicians and patients) in the co-design, field test, and evaluation of technologies supporting telerehabilitation services []. Accounting for the needs and preferences of patients in the early stages of program design can help mitigate concerns regarding home-based cardiac telerehabilitation component quality and can help in identifying issues with home-based cardiac telerehabilitation system quality program testing prior to trial implementation stages. However, we observed that less than one-fifth of home-based cardiac telerehabilitation programs reported including end users in the design and development stage and just over half undertook user testing of the home-based cardiac telerehabilitation system (Table S4 in ). American Heart Association’s recommendations on home-based cardiac telerehabilitation [] and the Beatty et al [] framework for mobile technology in cardiac rehabilitation can guide the development and evaluation of future home-based cardiac telerehabilitation programs.
Facilitating conditions, such as the timing of program introduction, prevailing weather conditions, and access to internet and computers, were reported to influence patients’ use of home-based cardiac telerehabilitation programs. Given that peak lifestyle changes occur in the first 6 months after diagnosis [] and that early cardiac rehabilitation is a significant predictor of cardiac function and functional capacity [,], home-based cardiac telerehabilitation should be offered as early as possible for patients to ensure optimal outcomes. Home-based cardiac telerehabilitation should also offer patients alternatives for either indoor or outdoor exercise training, especially in regions with seasonal weather changes. Additionally, as inequities in cardiovascular health still exist, an examination of socioeconomic characteristics are crucial if technology accessibility and affordability issues surrounding home-based cardiac telerehabilitation usage are to be addressed [,]. Access to technology infrastructure remains unevenly distributed worldwide, with internet use being significantly lower in low- and middle-income regions than in high-income regions []. Partnerships with local governing bodies should be established to marshal resources and secure funding to invest in required infrastructure []. Collaborating with nongovernment organizations to advocate for prospects on insurance coverage and to negotiate reasonable pricing and subsidies for mobile phones, data plans, and wearables will aid in supporting the long-term implementation and scale-up of home-based cardiac telerehabilitation in clinical practice [].
Although intrinsic factors such as lack of time and motivation are less amenable to change, program adaptations can be made to palliate concerns regarding data privacy, perceived technology self-efficacy and reliability, and preconceived age-related beliefs regarding home-based cardiac telerehabilitation usage. Program training, technological support, and the availability of transparent privacy policies, especially for older adults, can reduce potential uneasiness and facilitate willingness to engage in digital health technologies such as home-based cardiac telerehabilitation [,,]. Even though the majority of included home-based cardiac telerehabilitation provided face-to-face program training, less than one-third offered ongoing technological support during program intervention, and only half indicated using secure password-protected platforms (Table S4 in ). Future programs should develop introductory training sessions that are supported by practical step-by-step instruction manuals with designated technical support staff on home-based cardiac telerehabilitation platforms that comply with data governance regulations and security protocols to mitigate the risk of privacy breaches [,].
Limitations
This scoping review has some limitations that need to be acknowledged. First, the inclusion of only English-language papers may have resulted in the omission of eligible papers published in other languages. However, our comprehensive search strategy and broad inclusion of different study designs with no time restrictions allows for breadth and depth of inclusion in this review. Second, although the technology acceptance model offers a user-centered approach in mapping patient perspectives of home-based cardiac telerehabilitation program acceptance, content analysis is inherently reductive and could have limited the scope of our findings. However, the thematic analysis undertaken to explore the external variables influencing home-based cardiac telerehabilitation acceptance could have mitigated the risks of missing meaningful data from the studies included in our review. Lastly, although end users in this user-centered approach also include health care providers delivering home-based cardiac telerehabilitation, the evaluation of technology acceptance from provider perspectives was not included because it was not the focus of this review. It is likely that the underlying determinants of home-based cardiac telerehabilitation acceptance may differ in these users. We recommend that future research in the field of home-based cardiac telerehabilitation aim to include literature in other languages, utilize other available conceptual frameworks on digital health acceptance, and accommodate perspectives from different categories of end users in order to fully comprehend and address home-based cardiac telerehabilitation implementation and acceptance.
Conclusions
We drew on the technology acceptance model to map available research on patient’s technology acceptance of home-based cardiac telerehabilitation. Our results demonstrated that, while patient perspectives on home-based cardiac telerehabilitation usability, utility, acceptability, and acceptance were high, a number of external variables influence technology acceptance of home-based cardiac telerehabilitation programs. Additionally, gaps in current home-based cardiac telerehabilitation evaluation timing and approaches were revealed. As the appeal for home-based cardiac telerehabilitation grows during the COVID-19 pandemic and beyond, findings from this review can be used to provide guidance for stakeholders and clinicians in developing and evaluating patient-centered home-based cardiac telerehabilitation programs.
Acknowledgments
The assistance of Ms. Annelissa Chin with the peer-review database search strategy is greatly appreciated. This project received funding (grant MOH-000364) from the National Medical Research Council, under the Ministry of Health Singapore.
Conflicts of Interest
None declared.
Search strategy.
PDF File (Adobe PDF File), 131 KB
Summary, characteristics, and outcomes of studies.
PDF File (Adobe PDF File), 394 KBReferences
- Mensah GA, Roth GA, Fuster V. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J Am Coll Cardiol 2019 Nov 19;74(20):2529-2532 [FREE Full text] [CrossRef] [Medline]
- Ambrosetti M, Abreu A, Corrà U, Davos CH, Hansen D, Frederix I, et al. Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 update. a position paper from the secondary prevention and rehabilitation section of the European Association of Preventive Cardiology. Eur J Prev Cardiol 2020 Apr 07:2047487320913379. [CrossRef] [Medline]
- Santiago de Araújo Pio C, Chaves G, Davies P, Taylor R, Grace S. Interventions to promote patient utilisation of cardiac rehabilitation. Cochrane Database Syst Rev 2019 Feb 01;2:CD007131 [FREE Full text] [CrossRef] [Medline]
- Scherrenberg M, Wilhelm M, Hansen D, Völler H, Cornelissen V, Frederix I, et al. The future is now: a call for action for cardiac telerehabilitation in the COVID-19 pandemic from the secondary prevention and rehabilitation section of the European Association of Preventive Cardiology. Eur J Prev Cardiol 2020 Jul 02:2047487320939671. [CrossRef] [Medline]
- Ghisi GLDM, Xu Z, Liu X, Mola A, Gallagher R, Babu AS, et al. Impacts of the COVID-19 pandemic on cardiac rehabilitation delivery around the world. Glob Heart 2021 Jun 10;16(1):43-43 [FREE Full text] [CrossRef] [Medline]
- Thomas RJ, Beatty AL, Beckie TM, Brewer LC, Brown TM, Forman DE, et al. Home-based cardiac rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation 2019 Jul 02;140(1):e69-e89 [FREE Full text] [CrossRef] [Medline]
- Ramachandran H, Jiang Y, Tam W, Yeo T, Wang W. Effectiveness of home-based cardiac telerehabilitation as an alternative to phase 2 cardiac rehabilitation of coronary heart disease: a systematic review and meta-analysis. Eur J Prev Cardiol 2021 Jul 13 [FREE Full text] [CrossRef] [Medline]
- Frederix I, Caiani EG, Dendale P, Anker S, Bax J, Böhm A, et al. ESC e-Cardiology working group position paper: overcoming challenges in digital health implementation in cardiovascular medicine. Eur J Prev Cardiol 2019 Jul;26(11):1166-1177. [CrossRef] [Medline]
- Saarijärvi M, Wallin L, Bratt E. Process evaluation of complex cardiovascular interventions: how to interpret the results of my trial? Eur J Cardiovasc Nurs 2020 Mar;19(3):269-274 [FREE Full text] [CrossRef] [Medline]
- Gallagher R, Zhang L. Evaluating mobile health technologies: does the traditional randomized controlled trial serve our needs? Eur J Cardiovasc Nurs 2021 Aug 20;20(6):623-626. [CrossRef] [Medline]
- Davis FD, Venkatesh V. A critical assessment of potential measurement biases in the technology acceptance model: three experiments. Int J Hum Comput Stud 1996 Jul;45(1):19-45. [CrossRef]
- Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005 Feb 23;8(1):19-32. [CrossRef]
- Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol 2018 Dec 19;18(1):143-143 [FREE Full text] [CrossRef] [Medline]
- Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018 Oct 02;169(7):467-473 [FREE Full text] [CrossRef] [Medline]
- Nielsen J. Usability Engineering. San Francisco: Morgan Kaufmann; 1994.
- Whitelaw S, Pellegrini D, Mamas M, Cowie M, Van Spall HGC. Barriers and facilitators of the uptake of digital health technology in cardiovascular care: a systematic scoping review. Eur Heart J Digit Health 2021 Mar;2(1):62-74 [FREE Full text] [CrossRef] [Medline]
- Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs 2008 Mar 18;62(1):107-115. [CrossRef] [Medline]
- Sandelowski M. Qualitative analysis: what it is and how to begin. Res Nurs Health 1995 Aug 01;18(4):371-375. [CrossRef] [Medline]
- Kyngas H, Vanhanen L. Content analysis as a research method [Finnish]. Hoitotiede 1999;11:3-12.
- Devi R, Powell J, Singh S. A web-based program improves physical activity outcomes in a primary care angina population: randomized controlled trial. J Med Internet Res 2014 Sep 12;16(9):e186 [FREE Full text] [CrossRef] [Medline]
- Devi R, Carpenter C, Powell J, Singh S. Exploring the experience of using a web-based cardiac rehabilitation programme in a primary care angina population: a qualitative study. Int J Ther Rehabil 2014 Sep 02;21(9):434-440. [CrossRef]
- Ding EY, Erskine N, Stut W, McManus DD, Peterson A, Wang Z, et al. MI-PACE home-based cardiac telerehabilitation program for heart attack survivors: usability study. JMIR Hum Factors 2021 Jul 08;8(3):e18130 [FREE Full text] [CrossRef] [Medline]
- Dorje T, Zhao G, Tso K, Wang J, Chen Y, Tsokey L, et al. Smartphone and social media-based cardiac rehabilitation and secondary prevention in China (SMART-CR/SP): a parallel-group, single-blind, randomised controlled trial. Lancet Digit Health 2019 Nov;1(7):e363-e374 [FREE Full text] [CrossRef] [Medline]
- Fang J, Li J, Li ZH, Xu DM, Chen C, Xie B, et al. Attitudes towards acceptance of an innovative home-based and remote sensing rehabilitation protocol among cardiovascular patients in Shantou, China. J Geriatr Cardiol 2016 May;13(4):326-332 [FREE Full text] [CrossRef] [Medline]
- Harzand A, Witbrodt B, Davis-Watts ML, Alrohaibani A, Goese D, Wenger NK, et al. Feasibility of a smartphone-enabled cardiac rehabilitation program in male veterans with previous clinical evidence of coronary heart disease. Am J Cardiol 2018 Nov 01;122(9):1471-1476 [FREE Full text] [CrossRef] [Medline]
- Higgins RO, Rogerson M, Murphy BM, Navaratnam H, Butler MV, Barker L, et al. Cardiac rehabilitation online pilot: extending reach of cardiac rehabilitation. J Cardiovasc Nurs 2017;32(1):7-13. [CrossRef] [Medline]
- Rawstorn JC, Gant N, Rolleston A, Whittaker R, Stewart R, Benatar J, et al. End users want alternative intervention delivery models: usability and acceptability of the Remote-CR exercise-based cardiac telerehabilitation program. Arch Phys Med Rehabil 2018 Nov 01;99(11):2373-2377. [CrossRef] [Medline]
- Song Y, Ren C, Liu P, Tao L, Zhao W, Gao W. Effect of smartphone-based telemonitored exercise rehabilitation among patients with coronary heart disease. J Cardiovasc Transl Res 2019 Dec 9;13(4):659-667 [FREE Full text] [CrossRef] [Medline]
- Varnfield M, Karunanithi MK, Särelä A, Garcia E, Fairfull A, Oldenburg BF, et al. Uptake of a technology-assisted home-care cardiac rehabilitation program. Med J Aust 2011 Feb 21;194(4):S15-S19. [CrossRef] [Medline]
- Varnfield M, Karunanithi M, Lee C, Honeyman E, Arnold D, Ding H, et al. Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart 2014 Nov;100(22):1770-1779 [FREE Full text] [CrossRef] [Medline]
- Wang J, Zeng Z, Dong R, Sheng J, Lai Y, Yu J, et al. Efficacy of a WeChat based intervention to adherence to secondary prevention in patients undergoing coronary artery bypass graft in China: A randomized controlled trial. J Telemed Telecare 2020 Sep 30:1357633X20960639. [CrossRef] [Medline]
- Worringham C, Rojek A, Stewart I. Development and feasibility of a smartphone, ECG and GPS based system for remotely monitoring exercise in cardiac rehabilitation. PLoS One 2011 Feb 09;6(2):e14669 [FREE Full text] [CrossRef] [Medline]
- Yu C, Liu C, Du J, Liu H, Zhang H, Zhao Y, MISSION-2 Collaborative Group. Smartphone-based application to improve medication adherence in patients after surgical coronary revascularization. Am Heart J 2020 Oct;228:17-26. [CrossRef] [Medline]
- Yudi M, Clark D, Tsang D, Jelinek M, Kalten K, Joshi SB, et al. SMARTphone-based, early cardiac REHABilitation in patients with acute coronary syndromes: a randomized controlled trial. Coron Artery Dis 2021 Aug 01;32(5):432-440. [CrossRef] [Medline]
- Zutz A, Ignaszewski A, Bates J, Lear SA. Utilization of the internet to deliver cardiac rehabilitation at a distance: a pilot study. Telemed J E Health 2007 Jun;13(3):323-330. [CrossRef] [Medline]
- Lear SA, Singer J, Banner-Lukaris D, Horvat D, Park JE, Bates J, et al. Randomized trial of a virtual cardiac rehabilitation program delivered at a distance via the internet. Circ Cardiovasc Qual Outcomes 2014 Nov;7(6):952-959. [CrossRef] [Medline]
- Banner D, Lear S, Kandola D, Singer J, Horvat D, Bates J, et al. The experiences of patients undertaking a 'virtual' cardiac rehabilitation program. Stud Health Technol Inform 2015;209:9-14. [Medline]
- Nadal C, Sas C, Doherty G. Technology acceptance in mobile health: scoping review of definitions, models, and measurement. J Med Internet Res 2020 Jul 06;22(7):e17256 [FREE Full text] [CrossRef] [Medline]
- Wensing M, Grol R. Knowledge translation in health: how implementation science could contribute more. BMC Med 2019 May 07;17(1):88 [FREE Full text] [CrossRef] [Medline]
- Creswell J, Creswell J. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches Fifth ed. California: Sage Publications; 2018.
- Sousa V, Dunn Lopez K. Towards usable e-health. a systematic review of usability questionnaires. Appl Clin Inform 2017 May 10;8(2):470-490 [FREE Full text] [CrossRef] [Medline]
- Coorey G, Neubeck L, Mulley J, Redfern J. Effectiveness, acceptability and usefulness of mobile applications for cardiovascular disease self-management: systematic review with meta-synthesis of quantitative and qualitative data. Eur J Prev Cardiol 2018 Mar;25(5):505-521. [CrossRef] [Medline]
- Lee Y, Kozar KA, Larsen KR. The technology acceptance model: past, present, and future. Commun Assoc Inf Syst 2003;12(1):50. [CrossRef]
- Jiang X, Ming W, You JH. The cost-effectiveness of digital health interventions on the management of cardiovascular diseases: systematic review. J Med Internet Res 2019 Jun 17;21(6):e13166 [FREE Full text] [CrossRef] [Medline]
- Beatty AL, Fukuoka Y, Whooley MA. Using mobile technology for cardiac rehabilitation: a review and framework for development and evaluation. J Am Heart Assoc 2013 Nov 01;2(6):e000568 [FREE Full text] [CrossRef] [Medline]
- Chow CK, Jolly S, Rao-Melacini P, Fox KA, Anand SS, Yusuf S. Association of diet, exercise, and smoking modification with risk of early cardiovascular events after acute coronary syndromes. Circulation 2010 Feb 16;121(6):750-758. [CrossRef] [Medline]
- Prescott E, Eser P, Mikkelsen N, Holdgaard A, Marcin T, Wilhelm M, et al. Cardiac rehabilitation of elderly patients in eight rehabilitation units in western Europe: outcome data from the EU-CaRE multi-centre observational study. Eur J Prev Cardiol 2020 Nov;27(16):1716-1729. [CrossRef] [Medline]
- Giallauria F, Acampa W, Ricci F, Vitelli A, Maresca L, Mancini M, et al. Effects of exercise training started within 2 weeks after acute myocardial infarction on myocardial perfusion and left ventricular function: a gated SPECT imaging study. Eur J Prev Cardiol 2012 Dec;19(6):1410-1419. [CrossRef] [Medline]
- Oertelt-Prigione S, Maas AH. Health inequalities in secondary prevention. Eur J Prev Cardiol 2017 Jun;24(3_suppl):116-122. [CrossRef] [Medline]
- Niederseer D, Schmied C. Socioeconomic status matters: how can we individualize cardiac rehabilitation according to different socioeconomic needs? Eur J Prev Cardiol 2020 Jun 02:510-512. [CrossRef] [Medline]
- The state of mobile internet connectivity 2020. GSM Association. URL: https://www.gsma.com/r/wp-content/uploads/2020/09/GSMA-State-of-Mobile-Internet-Connectivity-Report-2020.pdf [accessed 2021-08-15]
- Kruse C, Betancourt J, Ortiz S, Valdes Luna SM, Bamrah IK, Segovia N. Barriers to the use of mobile health in improving health outcomes in developing countries: systematic review. J Med Internet Res 2019 Oct 09;21(10):e13263 [FREE Full text] [CrossRef] [Medline]
- Liu P, Astudillo K, Velez D, Kelley L, Cobbs-Lomax D, Spatz ES. Use of mobile health applications in low-income populations: a prospective study of facilitators and barriers. Circ Cardiovasc Qual Outcomes 2020 Sep;13(9):e007031 [FREE Full text] [CrossRef] [Medline]
- Redfern J. Can older adults benefit from smart devices, wearables, and other digital health options to enhance cardiac rehabilitation? Clin Geriatr Med 2019 Nov;35(4):489-497. [CrossRef] [Medline]
- Tsai HS, Rikard RV, Cotten SR, Shillair R. Senior technology exploration, learning, and acceptance (STELA) model: from exploration to use – a longitudinal randomized controlled trial. Educational Gerontology 2019 Nov 18;45(12):728-743. [CrossRef]
Edited by G Eysenbach; submitted 02.11.21; peer-reviewed by E van der Velde; comments to author 23.11.21; revised version received 24.11.21; accepted 27.11.21; published 07.01.22
Copyright©Hadassah Joann Ramachandran, Ying Jiang, Jun Yi Claire Teo, Tee Joo Yeo, Wenru Wang. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 07.01.2022.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research, is properly cited. The complete bibliographic information, a link to the original publication on https://www.jmir.org/, as well as this copyright and license information must be included.

